Differential expression of HSP90 isoforms and their correlations with clinicopathologic factors in patients with colorectal cancer
- PMID: 31933908
- PMCID: PMC6945153
Differential expression of HSP90 isoforms and their correlations with clinicopathologic factors in patients with colorectal cancer
Abstract
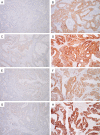
Heat shock protein 90 (HSP90), a molecular chaperone, plays critical roles in cellular protection against various stressful stimuli and in the regulation of cellular growth and apoptosis. HSP90 has four human isoforms; HSP90α, HSP90β, glucose related protein 94 (GRP94), and tumor necrosis factor (TNF) receptor-associated protein 1 (TRAP1). We evaluated the differential expression of these HSP90 isoforms in colorectal cancer (CRC) and correlated their expression levels with clinicopathological factors and patient survival rates. We performed immunohistochemical staining for HSP90α, HSP90β, GRP94, and TRAP1 in 129 CRC tumor samples and found that HSP90α expression was significantly associated with advanced pT stage (P = 0.011) and shorter recurrence-free survival (RFS) (P = 0.010), whereas GRP94 expression was correlated with low grade (P = 0.029) and better RFS (P < 0.001). HSP90β and TRAP1 had no prognostic impact, although HSP90β expression was positively correlated with tumor size (P = 0.008). Based on our results, HSP90α and GRP94 are potential prognostic biomarkers of CRC. In addition, the differences in expression and functional activities among four HSP90 isoforms imply that isoform selectivity should be seriously considered when HSP90 inhibitors are studied or adopted for the treatment of CRC.
Keywords: Colorectal cancer; GRP94; HSP90; TRAP1; immunohistochemistry; prognosis.
IJCEP Copyright © 2019.
Conflict of interest statement
None.
Figures

Similar articles
-
Differential expression of heat shock protein 90 isoforms in small cell lung cancer.Int J Clin Exp Pathol. 2015 Aug 1;8(8):9487-93. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26464709 Free PMC article.
-
The Differential Roles of HSP90 Isoforms in Skin Inflammation: Anti-Inflammatory Potential of TRAP1 Inhibition.J Invest Dermatol. 2025 Sep;145(9):2281-2292.e3. doi: 10.1016/j.jid.2025.02.006. Epub 2025 Feb 18. J Invest Dermatol. 2025. PMID: 39978584
-
Anti-inflammatory activities of novel heat shock protein 90 isoform selective inhibitors in BV-2 microglial cells.Front Mol Biosci. 2024 May 2;11:1405339. doi: 10.3389/fmolb.2024.1405339. eCollection 2024. Front Mol Biosci. 2024. PMID: 38756532 Free PMC article.
-
Organelle-specific Hsp90 inhibitors.Arch Pharm Res. 2015 Sep;38(9):1582-90. doi: 10.1007/s12272-015-0636-1. Epub 2015 Jul 22. Arch Pharm Res. 2015. PMID: 26195286 Review.
-
TRAP1: a viable therapeutic target for future cancer treatments?Expert Opin Ther Targets. 2017 Aug;21(8):805-815. doi: 10.1080/14728222.2017.1349755. Epub 2017 Jul 18. Expert Opin Ther Targets. 2017. PMID: 28664757 Review.
Cited by
-
Clinicopathologic and Prognostic Association of GRP94 Expression in Colorectal Cancer with Synchronous and Metachronous Metastases.Int J Mol Sci. 2021 Jun 30;22(13):7042. doi: 10.3390/ijms22137042. Int J Mol Sci. 2021. PMID: 34208855 Free PMC article.
-
Strong Hsp90α/β Protein Expression in Advanced Primary CRC Indicates Short Survival and Predicts Response to the Hsp90α/β-Specific Inhibitor Pimitespib.Cells. 2025 Jun 3;14(11):836. doi: 10.3390/cells14110836. Cells. 2025. PMID: 40498011 Free PMC article.
-
Cell Surface GRP94 as a Novel Emerging Therapeutic Target for Monoclonal Antibody Cancer Therapy.Cells. 2021 Mar 17;10(3):670. doi: 10.3390/cells10030670. Cells. 2021. PMID: 33802964 Free PMC article. Review.
-
BidSi6 and BidEL isoforms as a potential marker for predicting colorectal adenomatous polyps.BMC Med Genomics. 2022 Jun 6;15(1):129. doi: 10.1186/s12920-022-01282-0. BMC Med Genomics. 2022. PMID: 35668495 Free PMC article.
-
A clinically compatible drug-screening platform based on organotypic cultures identifies vulnerabilities to prevent and treat brain metastasis.EMBO Mol Med. 2022 Mar 7;14(3):e14552. doi: 10.15252/emmm.202114552. Epub 2022 Feb 17. EMBO Mol Med. 2022. PMID: 35174975 Free PMC article.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013, CA. Cancer J Clin. 2013;63:11–30. - PubMed
-
- Lim D, Ha M, Song I. Trends in major cancer mortality in Korea, 1983-2012, with a joinpoint analysis. Cancer Epidemiol. 2015;39:939–946. - PubMed
-
- Schlesinger MJ. Heat shock proteins. J Biol Chem. 1990;265:12111–12114. - PubMed
-
- Hendrick JP, Hartl FU. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993;62:349–384. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
