DLC-3 suppresses cellular proliferation, migration, and invasion in triple-negative breast cancer by the Wnt/β-catenin pathway
- PMID: 31933937
- PMCID: PMC6947060
DLC-3 suppresses cellular proliferation, migration, and invasion in triple-negative breast cancer by the Wnt/β-catenin pathway
Erratum in
-
Erratum: DLC-3 suppresses cellular proliferation, migration, and invasion in triple-negative breast cancer by the Wnt/β-catenin pathway.Int J Clin Exp Pathol. 2024 Nov 15;17(11):442-443. doi: 10.62347/WTHX6766. eCollection 2024. Int J Clin Exp Pathol. 2024. PMID: 39660331 Free PMC article.
Abstract
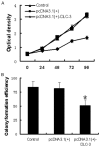
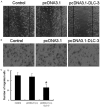
Triple-negative breast cancer (TNBC) is the most aggressive breast cancer subtype. Our study investigated the functional role of DLC-3 in TNBC. The expression of DLC-3 was assessed by immunohistochemistry in TNBC to evaluate the clinicopathologic significance of DLC-3. Recombinant lentiviral vectors encoding the DLC-3 gene were constructed for transfection into MDA-MB-231. Real-time qPCR and western blot analysis were employed to evaluate the expression of DLC-3, β-catenin, GSK-3β and c-myc in DLC-3-transfected cells. Moreover, cell proliferation assays, cell colony formation assays, and cell migration and invasion assays were performed to elucidate the role of DLC-3 in TNBC development and progression. Our data revealed that DLC-3 was downregulated in TNBC, and its expression level was associated with lymph node status and differentiation grade in breast cancer. Both real-time qPCR and western blot analyses showed that the DLC-3 gene and protein were overexpressed in the DLC-3-transfected MDA-MB-231 cells. In addition, the expression of GSK-3β was upregulated and the expression of β-catenin and c-myc gene was downregulated in the DLC-3-transfected cells. Furthermore, DLC-3 overexpression inhibited cell proliferation, colony formation, migration, and invasion in vitro. DLC-3, functioning as a tumor-suppressor gene, inhibits cell growth and invasion in TNBC, possibly through regulation of the Wnt/β-catenin signaling pathway.
Keywords: DLC-3; Triple-negative breast cancer; invasion; proliferation.
IJCEP Copyright © 2019.
Conflict of interest statement
None.
Figures



References
-
- Safonov A, Jiang T, Bianchini G, Győrffy B, Karn T, Hatzis C, Pusztai L. Immune gene expression is associated with genomic aberrations in breast cancer. Cancer Res. 2017;12:3317–3324. - PubMed
-
- Huang GH, Sun ZL, Li HJ, Feng DF. Rho GTPase-activating proteins: regulators of Rho GTPase activity in neuronal development and CNS diseases. Mol Cell Neurosci. 2017;80:18–31. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
