High mobility group protein B1 (HMGB1) interacts with receptor for advanced glycation end products (RAGE) to promote airway smooth muscle cell proliferation through ERK and NF- κ B pathways
- PMID: 31934170
- PMCID: PMC6949814
High mobility group protein B1 (HMGB1) interacts with receptor for advanced glycation end products (RAGE) to promote airway smooth muscle cell proliferation through ERK and NF- κ B pathways
Abstract
Background: High-mobility graoup box protein 1 (HMGB1) has been shown to mediate a wide range of pathologic responses by interacting with RAGE (receptor for advanced glycation endproducts) and TLRs (Toll-like receptors). Our previous study showed that HMGB1 has been involved in pathogenesis of airway remodeling in an allergen-induced chronic mice asthma model. Increased airway smooth muscle (ASM) mass is a vital feature of airway remodeling.
Objective: To evaluate the effect of HMGB1 on proliferation of ASMs and the underlying mechanisms.
Methods: Rat airway smooth muscle cells (RASMs) were obtained by primary explant techniques. We investigated the effect of HMGB1 on the proliferation of RASMs. To identify which receptors and signaling pathways be involved in proliferation of RASMs, we performed western blot and CCK-8 assay by specific receptor blockade and inhibition of MAPK (p38, JNK and ERK) and NF-κB signaling pathways.
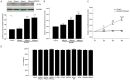
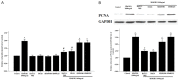
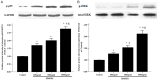
Results: HMGB1 stimulated RASMs proliferation in a dose- and time-dependent manner and also increased proliferating cell nuclear antigen (PCNA) and RAGE expression of RASMs. The inhibitor of RAGE, but not TLR2 and TLR4, reversed HMGB1-induced RASM proliferation and PCNA expression. Incubation of RASMs with HMGB1 caused a rapid increase in P65 and ERK phosphorylation. RASM proliferation and PCNA expression toward HMGB1 were significantly inhibited by the inhibitors of ERK and NF-κB.
Conclusion: HMGB1 induces proliferation of RASMs through a RAGE-dependent activation of ERK and NF-κB signaling pathways.
Keywords: HMGB1; RAGE; RASM cells; cell remodeling.
IJCEP Copyright © 2019.
Conflict of interest statement
None.
Figures



Similar articles
-
HMGB1 increases RAGE expression in vascular smooth muscle cells via ERK and p-38 MAPK-dependent pathways.Korean J Physiol Pharmacol. 2022 Sep 1;26(5):389-396. doi: 10.4196/kjpp.2022.26.5.389. Korean J Physiol Pharmacol. 2022. PMID: 36039739 Free PMC article.
-
[Role of HMGB1-RAGE/TLRs-NF-κB signaling pathway on bone mesenchymal stem cells transplantation therapy for lipopolysaccaride-induced coagulation disorder rats].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018 Sep;30(9):830-835. doi: 10.3760/cma.j.issn.2095-4352.2018.09.003. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018. PMID: 30309407 Chinese.
-
High-mobility group box-B1 (HMGB1) mediates the hypoxia-induced mesenchymal transition of osteoblast cells via activating ERK/JNK signaling.Cell Biol Int. 2016 Nov;40(11):1152-1161. doi: 10.1002/cbin.10616. Epub 2016 Sep 15. Cell Biol Int. 2016. PMID: 27106169
-
Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1).Angiogenesis. 2008;11(1):91-9. doi: 10.1007/s10456-008-9093-5. Epub 2008 Feb 9. Angiogenesis. 2008. PMID: 18264787 Review.
-
HMGB1/RAGE axis in tumor development: unraveling its significance.Front Oncol. 2024 Mar 1;14:1336191. doi: 10.3389/fonc.2024.1336191. eCollection 2024. Front Oncol. 2024. PMID: 38529373 Free PMC article. Review.
Cited by
-
Nε-(1-Carboxymethyl)-L-lysine/RAGE Signaling Drives Metastasis and Cancer Stemness through ERK/NFκB axis in Osteosarcoma.Int J Biol Sci. 2024 Jan 12;20(3):880-896. doi: 10.7150/ijbs.90817. eCollection 2024. Int J Biol Sci. 2024. PMID: 38250151 Free PMC article.
-
Protective effect of dexmedetomidine in cecal ligation perforation-induced acute lung injury through HMGB1/RAGE pathway regulation and pyroptosis activation.Bioengineered. 2021 Dec;12(2):10608-10623. doi: 10.1080/21655979.2021.2000723. Bioengineered. 2021. PMID: 34747306 Free PMC article.
-
Coffea arabica Extract Attenuates Atopic Dermatitis-like Skin Lesions by Regulating NLRP3 Inflammasome Expression and Skin Barrier Functions.Int J Mol Sci. 2023 Aug 2;24(15):12367. doi: 10.3390/ijms241512367. Int J Mol Sci. 2023. PMID: 37569742 Free PMC article.
-
Azilsartan Modulates HMGB1/NF-κB/p38/ERK1/2/JNK and Apoptosis Pathways during Renal Ischemia Reperfusion Injury.Cells. 2023 Jan 2;12(1):185. doi: 10.3390/cells12010185. Cells. 2023. PMID: 36611978 Free PMC article.
-
Thrombomodulin reduces α-synuclein generation and ameliorates neuropathology in a mouse model of Parkinson's disease.Cell Death Discov. 2024 Apr 8;10(1):167. doi: 10.1038/s41420-024-01939-y. Cell Death Discov. 2024. PMID: 38589400 Free PMC article.
References
-
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. - PubMed
-
- Parkkinen J, Raulo E, Merenmies J, Nolo R, Kajander EO, Baumann M, Rauvala H. Amphoterin, the 30-kDa protein in a family of HMG1-type polypeptides. Enhanced expression in transformed cells, leading edge localization, and interactions with plasminogen activation. J Biol Chem. 1993;268:19726–38. - PubMed
-
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7. - PubMed
-
- Bianchi ME, Crippa MP, Manfredi AA, Mezzapelle R, Rovere Querini P, Venereau E. High-mobility group box 1 protein orchestrates responses to tissue damage via inflammation, innate and adaptive immunity, and tissue repair. Immunol Rev. 2017;280:74–82. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
