Pro- and Antioxidant Effects of Vitamin C in Cancer in correspondence to Its Dietary and Pharmacological Concentrations
- PMID: 31934267
- PMCID: PMC6942884
- DOI: 10.1155/2019/7286737
Pro- and Antioxidant Effects of Vitamin C in Cancer in correspondence to Its Dietary and Pharmacological Concentrations
Abstract
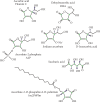
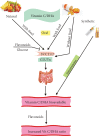
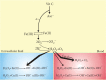
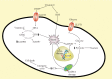
Vitamin C is an antioxidant that may scavenge reactive oxygen species preventing DNA damage and other effects important in cancer transformation. Dietary vitamin C from natural sources is taken with other compounds affecting its bioavailability and biological effects. High pharmacological doses of vitamin C may induce prooxidant effects, detrimental for cancer cells. An oxidized form of vitamin C, dehydroascorbate, is transported through glucose transporters, and cancer cells switch from oxidative phosphorylation to glycolysis in energy production so an excess of vitamin C may limit glucose transport and ATP production resulting in energetic crisis and cell death. Vitamin C may change the metabolomic and epigenetic profiles of cancer cells, and activation of ten-eleven translocation (TET) proteins and downregulation of pluripotency factors by the vitamin may eradicate cancer stem cells. Metastasis, the main reason of cancer-related deaths, requires breakage of anatomical barriers containing collagen, whose synthesis is promoted by vitamin C. Vitamin C induces degradation of hypoxia-inducible factor, HIF-1, essential for the survival of tumor cells in hypoxic conditions. Dietary vitamin C may stimulate the immune system through activation of NK and T cells and monocytes. Pharmacological doses of vitamin C may inhibit cancer transformation in several pathways, but further studies are needed to address both mechanistic and clinical aspects of this effect.
Copyright © 2019 Elzbieta Pawlowska et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Nishikimi M., Fukuyama R., Minoshima S., Shimizu N., Yagi K. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. The Journal of biological chemistry. 1994;269(18):13685–13688. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

