Comparison of treatment efficacy and safety between drug-eluting bead transarterial chemoembolization with CalliSpheres® microspheres and conventional transarterial chemoembolization as first-line treatment in hepatocellular carcinoma patients
- PMID: 31934293
- PMCID: PMC6943446
Comparison of treatment efficacy and safety between drug-eluting bead transarterial chemoembolization with CalliSpheres® microspheres and conventional transarterial chemoembolization as first-line treatment in hepatocellular carcinoma patients
Abstract
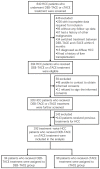
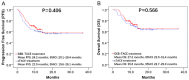
We aimed to compare the treatment response, survivals and safety of drug-eluting bead (DEB) transarterial chemoembolization (TACE) with CalliSpheres® microspheres (CSM) and conventional TACE (cTACE) as first-line treatment in Chinese HCC patients. 192 HCC patients from multiple centers received DEB-TACE with CSM or cTACE treatment as first-line treatment were included and assigned to DEB-TACE group (N=94) or cTACE group (N=98) accordingly. Treatment response was assessed at 1 month (M1), M3 and M6 after treatment. Progression-free survival (PFS) and overall survival (OS) was evaluated. Liver function indexes and adverse events were recorded. Complete response (CR) and objective response rate (ORR) were higher, while disease control rate (DCR) rate was similar in DEB-TACE group compared with cTACE group, and further multivariate logistic regression analysis validated that DEB-TACE vs cTACE independently predicted higher ORR. For survivals, no difference in PFS or OS was observed between DEB-TACE and cTACE groups, and multivariate Cox's proportional hazards regression revealed that DEB-TACE vs cTACE was not correlated with PFS or OS either. Additionally, no difference in liver function indexes at M1 or changes of liver function indexes from M0 to M1 between DEB-TACE and cTACE groups after treatment was observed, whereas DEB-TACE resulted in higher incidence of pain and fever during treatment or hospitalization. DEB-TACE with CSM discloses better treatment response, similar survival profiles and equal liver function injury but increased incidence of short-term adverse events than cTACE as the first-line therapy in treating HCC patients.
Keywords: DEB-TACE; HCC; safety; survival profiles; tumor response.
AJTR Copyright © 2019.
Conflict of interest statement
None.
Figures

References
-
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. - PubMed
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. - PubMed
-
- Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
