Pregnancy Outcome after Exposure to Migalastat for Fabry Disease: A Clinical Report
- PMID: 31934472
- PMCID: PMC6942789
- DOI: 10.1155/2019/1030259
Pregnancy Outcome after Exposure to Migalastat for Fabry Disease: A Clinical Report
Abstract
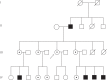
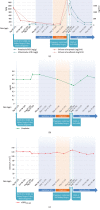
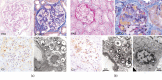
Our patient was a 37-year-old woman with Fabry disease (GLA p.R112H) with a medical history of recurrent headache, nausea, vomiting, vertigo, and tobacco use (20 cigarettes/day). Fabry disease was diagnosed in 2005 when she experienced proteinuria, preeclampsia, and hypertension (201/130 mm Hg) during pregnancy (delivered 50 cm, 3.4 kg healthy boy; GLA wild type [WT]). Enzyme replacement therapy was initiated in 2009. The patient enrolled in the phase 3 ATTRACT trial (ClinicalTrials.gov; NCT01218659) and started migalastat in May 2012 while taking hormonal contraceptives. Two years after initiating migalastat, the patient had proteinuria (2166 mg/24 h) without hypertension (131/68 mm Hg), which persisted (788 mg/24 h a month later). Kidney biopsy results were consistent with existing Fabry disease. A serum pregnancy test and ultrasound confirmed pregnancy (18 weeks' gestation). Migalastat and hormonal contraceptives were stopped; the patient continued to smoke. Fetal MRI was normal at ~29 weeks' gestation. In October 2014, at 37+ weeks' gestation, the patient delivered a 45-cm, 2.29-kg healthy girl (GLA WT). Excepting low birth weight, which may be related to the patient's smoking, pregnancy outcome was normal despite exposure to migalastat for 18 weeks. Migalastat therapy during pregnancy is not advised.
Copyright © 2019 Natalja Haninger-Vacariu et al.
Conflict of interest statement
Natalja Haninger-Vacariu has received travel grants from Amicus Therapeutics and Shire. Sarah El-Hadi, Udo Pauler, Marina Foretnik, Renate Kain, Alice Schmidt and Zoltán Prohászka declare that they have no conflicts of interest regarding the publication of this article. Nina Skuban and Jay A. Barth are employees of and hold stock in Amicus Therapeutics. Gere Sunder-Plassmann has served on advisory boards and received honoraria from Idorsia and Greenovation and honoraria and research funding from Amicus Therapeutics, Shire, and Sanofi.
Figures

Similar articles
-
Long-term efficacy and safety of migalastat treatment in Fabry disease: 30-month results from the open-label extension of the randomized, phase 3 ATTRACT study.Mol Genet Metab. 2020 Sep-Oct;131(1-2):219-228. doi: 10.1016/j.ymgme.2020.07.007. Epub 2020 Aug 15. Mol Genet Metab. 2020. PMID: 33012654 Clinical Trial.
-
Late-onset renal variant Fabry disease with R112H mutation and mild increase in plasma globotriaosylsphingosine: a case report.Front Med (Lausanne). 2024 Jun 6;11:1383309. doi: 10.3389/fmed.2024.1383309. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38903807 Free PMC article.
-
Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study.J Med Genet. 2017 Apr;54(4):288-296. doi: 10.1136/jmedgenet-2016-104178. Epub 2016 Nov 10. J Med Genet. 2017. PMID: 27834756 Free PMC article. Clinical Trial.
-
Migalastat: A Review in Fabry Disease.Drugs. 2019 Apr;79(5):543-554. doi: 10.1007/s40265-019-01090-4. Drugs. 2019. PMID: 30875019 Free PMC article. Review.
-
Does low-dose aspirin initiated before 11 weeks' gestation reduce the rate of preeclampsia?Am J Obstet Gynecol. 2020 May;222(5):437-450. doi: 10.1016/j.ajog.2019.08.047. Epub 2019 Sep 5. Am J Obstet Gynecol. 2020. PMID: 31494125
Cited by
-
Pregnancy outcomes of Fabry disease in Austria (PROFABIA)-a retrospective cohort-study.Orphanet J Rare Dis. 2024 Apr 18;19(1):165. doi: 10.1186/s13023-024-03180-3. Orphanet J Rare Dis. 2024. PMID: 38637893 Free PMC article.
References
-
- Ishii S., Chang H. H., Kawasaki K., et al. Mutant alpha-galactosidase a enzymes identified in Fabry disease patients with residual enzyme activity: biochemical characterization and restoration of normal intracellular processing by 1-deoxygalactonojirimycin. Biochemical Journal. 2007;406(2):285–295. doi: 10.1042/BJ20070479. - DOI - PMC - PubMed
-
- Schiffmann R., Hughes D. A., Linthorst G. E., et al. Screening, diagnosis, and management of patients with Fabry disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney International. 2017;91(2):284–293. doi: 10.1016/j.kint.2016.10.004. - DOI - PubMed
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical

