Effect of a Behavioral Intervention to Increase Vegetable Consumption on Cancer Progression Among Men With Early-Stage Prostate Cancer: The MEAL Randomized Clinical Trial
- PMID: 31935026
- PMCID: PMC6990696
- DOI: 10.1001/jama.2019.20207
Effect of a Behavioral Intervention to Increase Vegetable Consumption on Cancer Progression Among Men With Early-Stage Prostate Cancer: The MEAL Randomized Clinical Trial
Abstract
Importance: Guidelines endorsing vegetable-enriched diets to improve outcomes for prostate cancer survivors are based on expert opinion, preclinical studies, and observational data.
Objective: To determine the effect of a behavioral intervention that increased vegetable intake on cancer progression in men with early-stage prostate cancer.
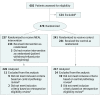
Design, setting, and participants: The Men's Eating and Living (MEAL) Study (CALGB 70807 [Alliance]) was a randomized clinical trial conducted at 91 US urology and medical oncology clinics that enrolled 478 men aged 50 to 80 years with biopsy-proven prostate adenocarcinoma (International Society of Urological Pathology grade group = 1 in those <70 years and ≤2 in those ≥70 years), stage cT2a or less, and serum prostate-specific antigen (PSA) level less than 10 ng/mL. Enrollment occurred from January 2011 to August 2015; 24-month follow-up occurred from January 2013 to August 2017.
Interventions: Patients were randomized to a counseling behavioral intervention by telephone promoting consumption of 7 or more daily vegetable servings (MEAL intervention; n = 237) or a control group, which received written information about diet and prostate cancer (n = 241).
Main outcomes and measures: The primary outcome was time to progression; progression was defined as PSA level of 10 ng/mL or greater, PSA doubling time of less than 3 years, or upgrading (defined as increase in tumor volume or grade) on follow-up prostate biopsy.
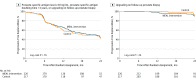
Results: Among 478 patients randomized (mean [SD] age, 64 [7] years; mean [SD] PSA level, 4.9 [2.1] ng/mL), 443 eligible patients (93%) were included in the primary analysis. There were 245 progression events (intervention: 124; control: 121). There were no significant differences in time to progression (unadjusted hazards ratio, 0.96 [95% CI, 0.75 to 1.24]; adjusted hazard ratio, 0.97 [95% CI, 0.76 to 1.25]). The 24-month Kaplan-Meier progression-free percentages were 43.5% [95% CI, 36.5% to 50.6%] and 41.4% [95% CI, 34.3% to 48.7%] for the intervention and control groups, respectively (difference, 2.1% [95% CI, -8.1% to 12.2%]).
Conclusions and relevance: Among men with early-stage prostate cancer managed with active surveillance, a behavioral intervention that increased vegetable consumption did not significantly reduce the risk of prostate cancer progression. The findings do not support use of this intervention to decrease prostate cancer progression in this population, although the study may have been underpowered to identify a clinically important difference.
Trial registration: ClinicalTrials.gov Identifier: NCT01238172.
Conflict of interest statement
Figures
Comment in
-
Re: Effect of a Behavioral Intervention to Increase Vegetable Consumption on Cancer Progression among Men with Early-Stage Prostate Cancer: The MEAL Randomized Clinical Trial.J Urol. 2020 Jul;204(1):185. doi: 10.1097/JU.0000000000001056. Epub 2020 Apr 15. J Urol. 2020. PMID: 32293960 No abstract available.
-
Vegetable Consumption and Progression of Prostate Cancer.JAMA. 2020 Jun 23;323(24):2528-2529. doi: 10.1001/jama.2020.6726. JAMA. 2020. PMID: 32573661 No abstract available.
-
Vegetable Consumption and Progression of Prostate Cancer.JAMA. 2020 Jun 23;323(24):2529-2530. doi: 10.1001/jama.2020.6729. JAMA. 2020. PMID: 32573662 No abstract available.
-
Re: Effect of a Behavioral Intervention to Increase Vegetable Consumption on Cancer Progression among Men with Early-Stage Prostate Cancer: The MEAL Randomized Clinical Trial.J Urol. 2020 Sep;204(3):608. doi: 10.1097/JU.0000000000001171. Epub 2020 Jun 26. J Urol. 2020. PMID: 32586177 No abstract available.
References
-
- Brooks JD, Paton VG, Vidanes G. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol Biomarkers Prev. 2001;10(9):949-954. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UG1 CA189974/CA/NCI NIH HHS/United States
- U10 CA041287/CA/NCI NIH HHS/United States
- UG1 CA189823/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
- R01 CA132951/CA/NCI NIH HHS/United States
- U10 CA077658/CA/NCI NIH HHS/United States
- U10 CA180830/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10 CA037447/CA/NCI NIH HHS/United States
- P30 CA023100/CA/NCI NIH HHS/United States
- UG1 CA233191/CA/NCI NIH HHS/United States
- U10 CA059518/CA/NCI NIH HHS/United States
- P30 CA016058/CA/NCI NIH HHS/United States
- U10 CA077651/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- U10 CA180866/CA/NCI NIH HHS/United States
- U10 CA138561/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

