Siponimod enriches regulatory T and B lymphocytes in secondary progressive multiple sclerosis
- PMID: 31935197
- PMCID: PMC7098784
- DOI: 10.1172/jci.insight.134251
Siponimod enriches regulatory T and B lymphocytes in secondary progressive multiple sclerosis
Abstract
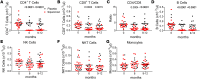
BACKGROUNDSiponimod (BAF312) is a selective sphingosine-1-phosphate receptor 1 and 5 (S1PR1, S1PR5) modulator recently approved for active secondary progressive multiple sclerosis (SPMS). The immunomodulatory effects of siponimod in SPMS have not been previously described.METHODSWe conducted a multicentered, randomized, double-blind, placebo-controlled AMS04 mechanistic study with 36 SPMS participants enrolled in the EXPAND trial. Gene expression profiles were analyzed using RNA derived from whole blood with Affymetrix Human Gene ST 2.1 microarray technology. We performed flow cytometry-based assays to analyze the immune cell composition and microarray gene expression analysis on peripheral blood from siponimod-treated participants with SPMS relative to baseline and placebo during the first-year randomization phase.RESULTSMicroarray analysis showed that immune-associated genes involved in T and B cell activation and receptor signaling were largely decreased by siponimod, which is consistent with the reduction in CD4+ T cells, CD8+ T cells, and B cells. Flow cytometric analysis showed that within the remaining lymphocyte subsets there was a reduction in the frequencies of CD4+ and CD8+ naive T cells and central memory cells, while T effector memory cells, antiinflammatory Th2, and T regulatory cells (Tregs) were enriched. Transitional regulatory B cells (CD24hiCD38hi) and B1 cell subsets (CD43+CD27+) were enriched, shifting the balance in favor of regulatory B cells over memory B cells. The proregulatory shift driven by siponimod treatment included a higher proliferative potential of Tregs compared with non-Tregs, and upregulated expression of PD-1 on Tregs. Additionally, a positive correlation was found between Tregs and regulatory B cells in siponimod-treated participants.CONCLUSIONThe shift toward an antiinflammatory and suppressive homeostatic immune system may contribute to the clinical efficacy of siponimod in SPMS.TRIAL REGISTRATIONNCT02330965.
Keywords: Autoimmunity; Multiple sclerosis.
Conflict of interest statement
Figures










References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

