The effect of storage conditions on microbial communities in stool
- PMID: 31935223
- PMCID: PMC6959592
- DOI: 10.1371/journal.pone.0227486
The effect of storage conditions on microbial communities in stool
Abstract
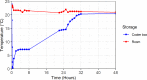
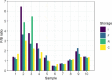
Microbiome research has experienced a surge of interest in recent years due to the advances and reduced cost of next-generation sequencing technology. The production of high quality and comparable data is dependent on proper sample collection and storage and should be standardized as far as possible. However, this becomes challenging when samples are collected in the field, especially in resource-limited settings. We investigated the impact of different stool storage methods common to the TB-CHAMP clinical trial on the microbial communities in stool. Ten stool samples were subjected to DNA extraction after 48-hour storage at -80°C, room temperature and in a cooler-box, as well as immediate DNA extraction. Three stool DNA extraction kits were evaluated based on DNA yield and quality. Quantitative PCR was performed to determine the relative abundance of the two major gut phyla Bacteroidetes and Firmicutes, and other representative microbial groups. The bacterial populations in the frozen group closely resembled the immediate extraction group, supporting previous findings that storage at -80°C is equivalent to the gold standard of immediate DNA extraction. More variation was seen in the room temperature and cooler-box groups, which may be due to the growth temperature preferences of certain bacterial populations. However, for most bacterial populations, no significant differences were found between the storage groups. As seen in other microbiome studies, the variation between participant samples was greater than that related to differences in storage. We determined that the risk of introducing bias to microbial community profiling through differences in storage will likely be minimal in our setting.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

