von Willebrand factor binding to myosin assists in coagulation
- PMID: 31935285
- PMCID: PMC6960459
- DOI: 10.1182/bloodadvances.2019000533
von Willebrand factor binding to myosin assists in coagulation
Abstract
von Willebrand factor (VWF) binds to platelets and collagen as a means of facilitating coagulation at sites of injury. Recent evidence has shown that myosin can serve as a surface for thrombin generation and binds to activated factor V and factor X. We studied whether VWF can also bind myosin as a means of bringing factor VIII (FVIII) to sites of clot formation. A myosin-binding assay was developed using skeletal muscle myosin to measure VWF binding, and plasma-derived and recombinant VWF containing molecular disruptions at key VWF sites were tested. Competition assays were performed using anti-VWF antibodies. FVIII binding to myosin was measured using a chromogenic FVIII substrate. Thrombin generation was measured using a fluorogenic substrate with and without myosin. Wild-type recombinant VWF and human plasma VWF from healthy controls bound myosin, whereas plasma lacking VWF exhibited no detectable myosin binding. Binding was multimer dependent and blocked by anti-VWF A1 domain antibodies or A1 domain VWF variants. The specific residues involved in myosin binding were similar, but not identical, to those required for collagen IV binding. FVIII did not bind myosin directly, but FVIII activity was detected when VWF and FVIII were bound to myosin. Myosin enhanced thrombin generation in platelet-poor plasma, although no difference was detected with the addition of myosin to platelet-rich plasma. Myosin may help to facilitate delivery of FVIII to sites of injury and indirectly accelerate thrombin generation by providing a surface for VWF binding in the setting of trauma and myosin exposure.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: R.R.M. has served as a consultant for Shire. The remaining authors declare no competing financial interests.
Figures

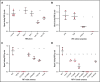
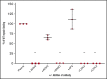
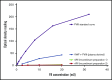
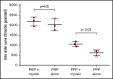
Similar articles
-
The physical exchange of factor VIII (FVIII) between von Willebrand factor and activated platelets and the effect of the FVIII B-domain on platelet binding.Biochemistry. 1997 Sep 2;36(35):10760-7. doi: 10.1021/bi970052+. Biochemistry. 1997. PMID: 9271507
-
Multimeric structure of platelet factor VIII/von Willebrand factor: the presence of larger multimers and their reassociation with thrombin-stimulated platelets.Blood. 1982 Nov;60(5):1132-8. Blood. 1982. PMID: 6982084
-
Role of factor VIII-von Willebrand factor and fibronectin in the interaction of platelets in flowing blood with monomeric and fibrillar human collagen types I and III.J Clin Invest. 1985 Feb;75(2):531-40. doi: 10.1172/JCI111729. J Clin Invest. 1985. PMID: 3919060 Free PMC article.
-
To serve and protect: The modulatory role of von Willebrand factor on factor VIII immunogenicity.Blood Rev. 2017 Sep;31(5):339-347. doi: 10.1016/j.blre.2017.07.001. Epub 2017 Jul 4. Blood Rev. 2017. PMID: 28716211 Review.
-
Factor VIII: A Dynamic Modulator of Hemostasis and Thrombosis in Trauma.Anesth Analg. 2023 May 1;136(5):894-904. doi: 10.1213/ANE.0000000000006356. Epub 2023 Apr 14. Anesth Analg. 2023. PMID: 37058725 Review.
Cited by
-
Fibronectin binding to von Willebrand factor occurs via the A1 domain.Res Pract Thromb Haemost. 2021 Jun 5;5(5):e12534. doi: 10.1002/rth2.12534. eCollection 2021 Jun. Res Pract Thromb Haemost. 2021. PMID: 34136746 Free PMC article.
-
Novel blood coagulation molecules: Skeletal muscle myosin and cardiac myosin.J Thromb Haemost. 2021 Jan;19(1):7-19. doi: 10.1111/jth.15097. Epub 2020 Oct 25. J Thromb Haemost. 2021. PMID: 32920971 Free PMC article. Review.
-
Skeletal muscle myosin and cardiac myosin attenuate heparin's antithrombin-dependent anticoagulant activity.J Thromb Haemost. 2021 Feb;19(2):470-477. doi: 10.1111/jth.15169. Epub 2020 Dec 10. J Thromb Haemost. 2021. PMID: 33176060 Free PMC article.
-
circ_0001274 Competitively Binds miR-143-3p to Upregulate VWF Expression to Improve Acute Traumatic Coagulopathy.Oxid Med Cell Longev. 2023 Jan 31;2023:9650323. doi: 10.1155/2023/9650323. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 36760352 Free PMC article.
References
-
- Fujimura Y, Titani K, Holland LZ, et al. . von Willebrand factor. A reduced and alkylated 52/48-kDa fragment beginning at amino acid residue 449 contains the domain interacting with platelet glycoprotein Ib. J Biol Chem. 1986;261(1):381-385. - PubMed
-
- Pareti FI, Niiya K, McPherson JM, Ruggeri ZM. Isolation and characterization of two domains of human von Willebrand factor that interact with fibrillar collagen types I and III. J Biol Chem. 1987;262(28):13835-13841. - PubMed
-
- Nichols WL, Hultin MB, James AH, et al. . von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia. 2008;14(2):171-232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

