Stability and bioactivity of pepCD47 attachment on stainless steel surfaces
- PMID: 31935523
- PMCID: PMC7295035
- DOI: 10.1016/j.actbio.2019.12.039
Stability and bioactivity of pepCD47 attachment on stainless steel surfaces
Abstract
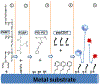
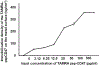
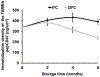
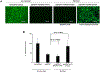
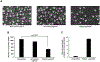
In-stent restenosis (ISR) and late stent thrombosis are the major complications associated with the use of metal stents and drug eluting stents respectively. Our lab previously investigated the use of peptide CD47 in improving biocompatibility of bare metal stents in a rat carotid stent model and our results demonstrated a significant reduction in platelet deposition and ISR. However, this study did not characterize the stability of the pepCD47 on metal surfaces post storage, sterilization and deployment. Thus, the objective of the present study was 1) to test the stability of the peptide post - storage, sterilization, exposure to shear and mechanical stress and 2) to begin to expand our current knowledge of pepCD47 coated metal surfaces into the preclinical large animal rabbit model. Our results show that the maximum immobilization density of pepCD47 on metal surfaces is approximately 350 ng/cm2. 100% of the pepCD47 was retained on the metal surface post 24 weeks of storage at 4 °C, exposure to physiological shear stress, and mechanical stress of stent expansion. The bioactivity of the pepCD47 was found to be intact post 24 weeks of storage and ethylene oxide sterilization. Finally our ex vivo studies demonstrated that compared to bare metal the rabbit pepCD47 coated surfaces showed - 45% reduced platelet adhesion, a 10-fold decrease in platelet activation, and 93% endothelial cell retention. Thus, our data suggests that pepCD47 coating on metal surfaces is stable and rabbit pepCD47 shows promising preliminary results in preventing thrombosis and not inhibiting the growth of endothelial cells. STATEMENT OF SIGNIFICANCE: Biocompatibility of bare metal stents is a major challenge owing to the significantly high rates of in-stent restenosis. Previously we demonstrated that peptide CD47 functionalization improves the biocompatibility of bare metal stents in rat model. A similar trend was observed in our ex vivo studies where rabbit blood was perfused over the rabbit pepCD47 functionalized surfaces. These results provide valuable proof of concept data for future in vivo rabbit model studies. In addition, we investigated stability of the pepCD47 on metal surface and observed that pepCD47 coating is stable over time and resistant to industrially relevant pragmatic challenges.
Keywords: CD47; Cell activation; Cell attachment; Peptide; Stability; Surface modification.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Benjamin EJ , Virani SS , Callaway CW , Chamberlain AM , Chang AR , Cheng S , Chiuve SE , Cushman M , Delling FN , Deo R , de Ferranti SD , Ferguson JF , Fornage M , Gillespie C , Isasi CR , Jimenez MC , Jordan LC , Judd SE , Lackland D , Lichtman JH , Lisabeth L , Liu S , Longenecker CT , Lutsey PL , Mackey JS , Matchar DB , Matsushita K , Mussolino ME , Nasir K , O’Flaherty M , Palaniappan LP , Pandey A , Pandey DK , Reeves MJ , Ritchey MD , Rodriguez CJ , Roth GA , Rosamond WD , Sampson UKA , Satou GM , Shah SH , Spartano NL , Tirschwell DL , Tsao CW , Voeks JH , Willey JZ , Wilkins JT , Wu JH , Alger HM , Wong SS , Muntner P , Heart disease and stroke statistics-2018 update: a report from the american heart association, Circulation 137 (12) (2018) e67–e492 . - PubMed
-
- Iqbal J , Gunn J , Serruys PW , Coronary stents: historical development, current status and future directions, Br. Med. Bull 106 (2013) 193–211 . - PubMed
-
- Hoffmann R , Mintz GS , Dussaillant GR , Popma JJ , Pichard AD , Satler LF , Kent KM , Griffin J , Leon MB , Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study, Circulation 94 (6) (1996) 1247–1254 . - PubMed
-
- Stefanini GG , Windecker S , Stent thrombosis: no longer an issue with newer–generation drug-eluting stents? Circ. Cardiovasc. Interv 5 (3) (2012) 332–335 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

