Emerging strategies for expanding the toolbox of enzymes in biocatalysis
- PMID: 31935627
- PMCID: PMC7769163
- DOI: 10.1016/j.cbpa.2019.12.006
Emerging strategies for expanding the toolbox of enzymes in biocatalysis
Abstract
Expanding the repertoire of reactions available to enzymes is an enduring challenge in biocatalysis. Owing to the synthetic versatility of transition metals, metalloenzymes have been favored targets for achieving new catalytic functions. Although less well explored, enzymes lacking metal centers can also be effective catalysts for non-natural reactions, providing access to reaction modalities that compliment those available to metals. By understanding how these activation modes can reveal new functions, strategies can be developed to access novel biocatalytic reactions. This review will cover discoveries in the last two years which access catalytic reactions that go beyond the native repertoire of metal-free biocatalysts.
Copyright © 2019. Published by Elsevier Ltd.
Conflict of interest statement
Conflict of interest statement Nothing declared.
Figures

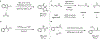
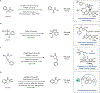
References
-
- Choi JM, Han SS, Kim HS, Industrial applications of enzyme biocatalysis: current status and future aspects, Biotechnol. Adv 33 (2015) 1443–1454. - PubMed
-
- Adams JP, Brown MJB, Diaz-Rodriguez A, Lloyd RC, Roiban G, Biocatalysis: A Pharma Perspective, Adv. Synth. Catal 361 (2019) 2421.
-
- Sheldon RA, Woodley JM, Role of Biocatalysis in Sustainable Chemistry, Chem. Rev 118 (2018) 801–838. - PubMed
-
- Turner NJ, O’Reily E, Biocatalytic retrosynthesis, Nat. Chem. Biol 9 (2013) 285–288. - PubMed
-
- Bornscheuer UT, Kazlauskas RJ, Catalytic promiscuity in biocatalysis: using old enzymes to form new bonds and follow new pathways, Angew. Chem. Int. Ed 43 (2004) 6032–6040. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

