Mass Spectrometry Imaging of Specialized Metabolites for Predicting Lichen Fitness and Snail Foraging
- PMID: 31935813
- PMCID: PMC7020473
- DOI: 10.3390/plants9010070
Mass Spectrometry Imaging of Specialized Metabolites for Predicting Lichen Fitness and Snail Foraging
Abstract
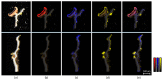
Lichens are slow-growing organisms supposed to synthetize specialized metabolites to protect themselves against diverse grazers. As predicted by the optimal defense theory (ODT), lichens are expected to invest specialized metabolites in higher levels in reproductive tissues compared to thallus. We investigated whether Laser Desorption Ionization coupled to Mass Spectrometry Imaging (LDI-MSI) could be a relevant tool for chemical ecology issues such as ODT. In the present study, this method was applied to cross-sections of thalli and reproductive tissues of the lichen Pseudocyphellaria crocata. Spatial mapping revealed phenolic families of metabolites. A quantification of these metabolites was carried out in addition to spatial imaging. By this method, accumulation of specialized metabolites was observed in both reproductive parts (apothecia and soralia) of P. crocata, but their nature depended on the lichen organs: apothecia concentrated norstictic acid, tenuiorin, and pulvinic acid derivatives, whereas soralia mainly contained tenuiorin and pulvinic acid. Stictic acid, tenuiorin and calycin, tested in no-choices feeding experiments, were deterrent for N. hookeri while entire thalli were consumed by the snail. To improve better knowledge in relationships between grazed and grazing organisms, LDI-MSI appears to be a complementary tool in ecological studies.
Keywords: Chemical Ecology; Lichens; Lobariaceae; Mass Spectrometry Imaging; Notodiscus hookeri; Optimal Defense Theory; Pseudocyphellaria crocata; Specialized Metabolites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


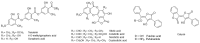

References
-
- Lawrey J.D. Chemical defense in lichen symbioses. In: White J.F., Torres M.S., editors. Defensive Mutualism in Microbial Symbiosis. CRC Press; Boca Raton, FL, USA: 2009. pp. 167–176.
-
- Schupp P., Eder C., Paul V., Proksch P. Distribution of secondary metabolites in the sponge Oceanapia sp. and its ecological implications. Mar. Biol. 1999;135:573–580. doi: 10.1007/s002270050658. - DOI
-
- Kliebenstein D. Secondary metabolites and plant/environment interactions: A view through Arabidopsis thaliana tinged glasses. Plant Cell Environ. 2004;27:675–684. doi: 10.1111/j.1365-3040.2004.01180.x. - DOI
-
- McKey D. Adaptive patterns in alkaloid physiology. Am. Nat. 1974;108:305–320. doi: 10.1086/282909. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

