Targeted Nano-Drug Delivery of Colchicine against Colon Cancer Cells by Means of Mesoporous Silica Nanoparticles
- PMID: 31936103
- PMCID: PMC7017376
- DOI: 10.3390/cancers12010144
Targeted Nano-Drug Delivery of Colchicine against Colon Cancer Cells by Means of Mesoporous Silica Nanoparticles
Abstract
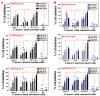
Antimitotics are important anticancer agents and include the natural alkaloid prodrug colchicine (COL). However, a major challenge of using COL as an anticancer drug is its cytotoxicity. We developed a novel drug delivery system (DDS) for COL using mesoporous silica nanoparticles (MSNs). The MSNs were functionalized with phosphonate groups, loaded with COL, and coated with folic acid chitosan-glycine complex. The resulting nanoformulation, called MSNsPCOL/CG-FA, was tested for action against cancer and normal cell lines. The anticancer effect was highly enhanced for MSNsPCOL/CG-FA compared to COL. In the case of HCT116 cells, 100% inhibition was achieved. The efficiency of MSNsPCOL/CG-FA ranked in this order: HCT116 (colon cancer) > HepG2 (liver cancer) > PC3 (prostate cancer). MSNsPCOL/CG-FA exhibited low cytotoxicity (4%) compared to COL (~60%) in BJ1 normal cells. The mechanism of action was studied in detail for HCT116 cells and found to be primarily intrinsic apoptosis caused by an enhanced antimitotic effect. Furthermore, a contribution of genetic regulation (metastasis-associated lung adenocarcinoma transcript 1 (MALAT 1), and microRNA (mir-205)) and immunotherapy effects (angiopoietin-2 (Ang-2 protein) and programmed cell death protein 1 (PD-1) was found. Therefore, this study shows enhanced anticancer effects and reduced cytotoxicity of COL with targeted delivery compared to free COL and is a novel method of developing cancer immunotherapy using a low-cost small-molecule natural prodrug.
Keywords: PD-1 immune checkpoint inhibitor and cancer immunotherapy; apoptosis; colchicine alkaloid; colon cancer cells; mesoporous silica nanoparticles; targeted delivery system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Cohen A.S., Rubinow A., Anderson J.J., Skinner M., Mason J.H., Libbey C., Kayne H. Survival of patients with primary (AL) amyloidosis: Colchicine-treated cases from 1976 to 1983 compared with cases seen in previous years (1961 to 1973) Am. J. Med. 1987;82:1182–1190. doi: 10.1016/0002-9343(87)90222-1. - DOI - PubMed
-
- Sampedro-Núñez M., Serrano-Somavilla A., Adrados M., Cameselle-Teijeiro J.M., Blanco-Carrera C., Cabezas-Agricola J.M., Martínez-Hernández R., Martín-Pérez E., Muñoz de Nova J.L., Díaz J.Á., et al. Analysis of expression of the PD-1/PD-L1 immune checkpoint system and its prognostic impact in gastroenteropancreatic neuroendocrine tumors. Sci. Rep. 2018;8:17812. doi: 10.1038/s41598-018-36129-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

