The Aryl Hydrocarbon Receptor Is Expressed in Thyroid Carcinoma and Appears to Mediate Epithelial-Mesenchymal-Transition
- PMID: 31936153
- PMCID: PMC7016998
- DOI: 10.3390/cancers12010145
The Aryl Hydrocarbon Receptor Is Expressed in Thyroid Carcinoma and Appears to Mediate Epithelial-Mesenchymal-Transition
Abstract
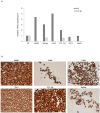
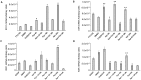
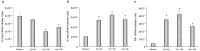
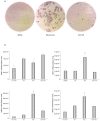
Aryl hydrocarbon receptor (AhR) is expected to promote initiation, progression and invasion of cancer cells regulating proliferation, differentiation, gene expression, inflammation, cell motility and migration. Furthermore, an immunosuppressant function of AhR has been recognized. This study evaluated AhR expression and its role in thyroid cancer progression. AhR expression was assessed by qPCR in 107 thyroid cancer samples (90 PTCs, 11 MTCs, 6 ATCs), and by immunohistochemistry in 41 PTCs. To estimate receptor activation, the expression of target genes CYP1A1 and CYP1B1 was measured. AhR functional effects were evaluated in kynurenine-stimulated FTC-133 and BcPap cell lines by analyzing the expression of genes involved in EMT and cell motility. AhR mRNA expression resulted significantly higher in all the analyzed thyroid cancer samples compared to normal thyroid and a statistically significant correlation with CYP1B1 was detected. Kynurenine-stimulated FTC-133 and BcPap showed the activation of a specific AhR-driven EMT program characterized by E-cadherin decrease and SLUG, N-cadherin and fibronectin increase, resulting in boost of cell motility and invasion. This study confirmed the importance of the IDO1-Kyn-AhR pathway in thyroid cancer tumorigenesis, suggesting an AhR pivotal role in mediating an immunosuppressive microenvironment and favoring the acquisition of a mesenchymal phenotype that could promote invasiveness and metastasis.
Keywords: AhR; EMT; SLUG; cell migration and invasiveness; thyroid cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



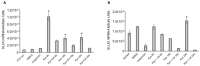




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

