Roles of TrkC Signaling in the Regulation of Tumorigenicity and Metastasis of Cancer
- PMID: 31936239
- PMCID: PMC7016819
- DOI: 10.3390/cancers12010147
Roles of TrkC Signaling in the Regulation of Tumorigenicity and Metastasis of Cancer
Abstract
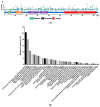
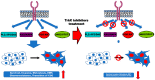
Tropomyosin receptor kinase (Trk) C contributes to the clinicopathology of a variety of human cancers, and new chimeric oncoproteins containing the tyrosine kinase domain of TrkC occur after fusion to the partner genes. Overexpression of TrkC and TrkC fusion proteins was observed in patients with a variety of cancers, including mesenchymal, hematopoietic, and those of epithelial cell lineage. Both microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) were involved in the regulation of TrkC expression through transcriptional and posttranscriptional alteration. Aberrant activation of TrkC and TrkC fusion proteins markedly induces the epithelial-mesenchymal transition (EMT) program, growth rate, tumorigenic capacity via constitutive activation of Ras-MAP kinase (MAPK), PI3K-AKT, and the JAK2-STAT3 pathway. The clinical trial of TrkC or TrkC fusion-positive cancers with newly developed Trk inhibitors demonstrated that Trk inhibitors were highly effective in inducing tumor regression in patients who do not harbor mutations in the kinase domain. Recently, there has been a progressive accumulation of mutations in TrkC or the TrkC fusion protein detected in the clinic and its related cancer cell lines caused by high-throughput DNA sequencing. Despite given the high overall response rate against Trk or Trk fusion proteins-positive solid tumors, acquired drug resistance was observed in patients with various cancers caused by mutations in the Trk kinase domain. To overcome acquired resistance caused by kinase domain mutation, next-generation Trk inhibitors have been developed, and these inhibitors are currently under investigation in clinical trials.
Keywords: TrkC; TrkC fusion; TrkC inhibitor; somatic mutation; targeted therapies.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
-
- Urfer R., Tsoulfas P., O’Connell L., Hongo J.A., Zhao W., Presta L.G. High resolution mapping of the binding site of TrkA for nerve growth factor and TrkC for neurotrophin-3 on the second immunoglobulin-like domain of the Trk receptors. J. Biol. Chem. 1998;273:5829–5840. doi: 10.1074/jbc.273.10.5829. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

