Expression and Localization of Kv1.1 and Kv3.1b Potassium Channels in the Cochlear Nucleus and Inferior Colliculus after Long-Term Auditory Deafferentation
- PMID: 31936259
- PMCID: PMC7017294
- DOI: 10.3390/brainsci10010035
Expression and Localization of Kv1.1 and Kv3.1b Potassium Channels in the Cochlear Nucleus and Inferior Colliculus after Long-Term Auditory Deafferentation
Abstract
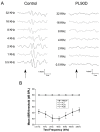
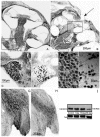
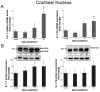
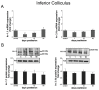
Deafness affects the expression and distribution of voltage-dependent potassium channels (Kvs) of central auditory neurons in the short-term, i.e., hours to days, but the consequences in the expression of Kvs after long-term deafness remain unknown. We tested expression and distribution of Kv1.1 and Kv3.1b, key for auditory processing, in the rat cochlear nucleus (CN), and in the inferior colliculus (IC), at 1, 15 and 90 days after mechanical lesion of the cochlea, using a combination of qRT-PCR and Western blot in the whole CN, along with semi-quantitative immunocytochemistry in the AVCN, where the role of both Kvs in excitability control for accurate auditory timing signal processing is well established. Neither Kv1.1/Kv3.1b mRNA or protein expression changed significantly in the CN between 1 and 15 days after deafness. At 90 days post-lesion, however, mRNA and protein expression for both Kvs increased, suggesting that expression regulation of Kv1.1 and Kv3.1b is part of cellular mechanisms for long-term adaptation to auditory input deprivation in the CN. Consistent with these findings, immunocytochemical localization showed increased labeling intensity for both Kvs in the AVCN at day 90 after cochlear lesion, further supporting that up-regulation of Kv1.1 and Kv3.1b in neurons of this CN division, over a long term after auditory deprivation, may be required to adapt intrinsic excitability to altered input. Contrary to findings in the CN, in the IC, expression levels of Kv1.1 and Kv3.1b did not undergo major changes after cochlear lesion. In particular, there was no evidence of long-term up-regulation of neither Kv1.1 or Kv3.1b, supporting that such post-lesion adaptive mechanism may not be needed in the IC. This suggests that post-lesion plastic adaptations to auditory input deprivation are not stereotypical along the auditory pathway.
Keywords: auditory; hearing loss; ion channels; plasticity; post-lesion plasticity.
Conflict of interest statement
This research was conducted in the absence of any commercial or financial interests that may be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

