Strenuous Acute Exercise Induces Slow and Fast Twitch-Dependent NADPH Oxidase Expression in Rat Skeletal Muscle
- PMID: 31936265
- PMCID: PMC7022445
- DOI: 10.3390/antiox9010057
Strenuous Acute Exercise Induces Slow and Fast Twitch-Dependent NADPH Oxidase Expression in Rat Skeletal Muscle
Abstract
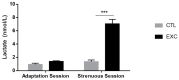
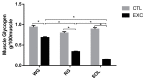
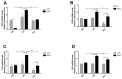
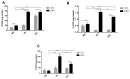
The enzymatic complex Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase (NOx) may be the principal source of reactive oxygen species (ROS). The NOX2 and NOX4 isoforms are tissue-dependent and are differentially expressed in slow-twitch fibers (type I fibers) and fast-twitch fibers (type II fibers) of skeletal muscle, making them different markers of ROS metabolism induced by physical exercise. The aim of this study was to investigate NOx signaling, as a non-adaptive and non-cumulative response, in the predominant fiber types of rat skeletal muscles 24 h after one strenuous treadmill exercise session. The levels of mRNA, reduced glycogen, thiol content, NOx, superoxide dismutase, catalase, glutathione peroxidase activity, and PPARGC1α and SLC2A4 gene expression were measured in the white gastrocnemius (WG) portion, the red gastrocnemius (RG) portion, and the soleus muscle (SOL). NOx activity showed higher values in the SOL muscle compared to the RG and WG portions. The same was true of the NOX2 and NOX4 mRNA levels, antioxidant enzymatic activities, glycogen content. Twenty-four hours after the strenuous exercise session, NOx expression increased in slow-twitch oxidative fibers. The acute strenuous exercise condition showed an attenuation of oxidative stress and an upregulation of antioxidant activity through PPARGC1α gene activity, antioxidant defense adaptations, and differential gene expression according to the predominant fiber type. The most prominent location of detoxification (indicated by NOX4 activation) in the slow-twitch oxidative SOL muscle was the mitochondria, while the fast-twitch oxidative RG portion showed a more cytosolic location. Glycolytic metabolism in the WG portion suggested possible NOX2/NOX4 non-regulation, indicating other possible ROS regulation pathways.
Keywords: antioxidant defenses; glycolytic metabolism fibers; oxidative metabolism fibers; reactive oxygen species; skeletal muscle fibers.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
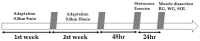
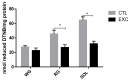

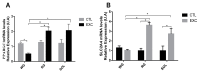
Similar articles
-
Differential Expression of NADPH Oxidases Depends on Skeletal Muscle Fiber Type in Rats.Oxid Med Cell Longev. 2016;2016:6738701. doi: 10.1155/2016/6738701. Epub 2016 Oct 26. Oxid Med Cell Longev. 2016. PMID: 27847553 Free PMC article.
-
Muscle fiber type comparison of PDH kinase activity and isoform expression in fed and fasted rats.Am J Physiol Regul Integr Comp Physiol. 2001 Mar;280(3):R661-8. doi: 10.1152/ajpregu.2001.280.3.R661. Am J Physiol Regul Integr Comp Physiol. 2001. PMID: 11171643
-
Skeletal muscle fiber type comparison of pyruvate dehydrogenase phosphatase activity and isoform expression in fed and food-deprived rats.Am J Physiol Endocrinol Metab. 2007 Feb;292(2):E571-6. doi: 10.1152/ajpendo.00327.2006. Epub 2006 Oct 3. Am J Physiol Endocrinol Metab. 2007. PMID: 17018773
-
Redox Profile of Skeletal Muscles: Implications for Research Design and Interpretation.Antioxidants (Basel). 2023 Sep 7;12(9):1738. doi: 10.3390/antiox12091738. Antioxidants (Basel). 2023. PMID: 37760040 Free PMC article. Review.
-
Muscle mechanics: adaptations with exercise-training.Exerc Sport Sci Rev. 1996;24:427-73. Exerc Sport Sci Rev. 1996. PMID: 8744258 Review.
Cited by
-
Nicotinamide: Oversight of Metabolic Dysfunction Through SIRT1, mTOR, and Clock Genes.Curr Neurovasc Res. 2020;17(5):765-783. doi: 10.2174/1567202617999201111195232. Curr Neurovasc Res. 2020. PMID: 33183203 Free PMC article. Review.
-
Targeting Hydrogen Sulfide Modulates Dexamethasone-Induced Muscle Atrophy and Microvascular Rarefaction, through Inhibition of NOX4 and Induction of MGF, M2 Macrophages and Endothelial Progenitors.Cells. 2022 Aug 11;11(16):2500. doi: 10.3390/cells11162500. Cells. 2022. PMID: 36010575 Free PMC article.
-
Glutathione Participation in the Prevention of Cardiovascular Diseases.Antioxidants (Basel). 2021 Jul 29;10(8):1220. doi: 10.3390/antiox10081220. Antioxidants (Basel). 2021. PMID: 34439468 Free PMC article. Review.
-
The Effect of Acute Aerobic Exercise on Redox Homeostasis and Mitochondrial Function of Rat White Adipose Tissue.Oxid Med Cell Longev. 2021 Jan 31;2021:4593496. doi: 10.1155/2021/4593496. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33603946 Free PMC article.
-
Effects of exercise on different antioxidant enzymes and related indicators: a systematic review and meta-analysis of randomized controlled trials.Sci Rep. 2025 Apr 11;15(1):12518. doi: 10.1038/s41598-025-97101-4. Sci Rep. 2025. PMID: 40216934 Free PMC article.
References
-
- Sakellariou G.K., Vasilaki A., Palomero J., Kaiany A., Zibrik L., Mcardle A., Jackson M.J. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid. Redox Signal. 2013;18:603–621. doi: 10.1089/ars.2012.4623. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

