Antibody Conjugates-Recent Advances and Future Innovations
- PMID: 31936270
- PMCID: PMC7148502
- DOI: 10.3390/antib9010002
Antibody Conjugates-Recent Advances and Future Innovations
Abstract
Monoclonal antibodies have evolved from research tools to powerful therapeutics in the past 30 years. Clinical success rates of antibodies have exceeded expectations, resulting in heavy investment in biologics discovery and development in addition to traditional small molecules across the industry. However, protein therapeutics cannot drug targets intracellularly and are limited to soluble and cell-surface antigens. Tremendous strides have been made in antibody discovery, protein engineering, formulation, and delivery devices. These advances continue to push the boundaries of biologics to enable antibody conjugates to take advantage of the target specificity and long half-life from an antibody, while delivering highly potent small molecule drugs. While the "magic bullet" concept produced the first wave of antibody conjugates, these entities were met with limited clinical success. This review summarizes the advances and challenges in the field to date with emphasis on antibody conjugation, linker-payload chemistry, novel payload classes, absorption, distribution, metabolism, and excretion (ADME), and product developability. We discuss lessons learned in the development of oncology antibody conjugates and look towards future innovations enabling other therapeutic indications.
Keywords: ADC; ADME; antibodies; antibody-drug conjugates; bioconjugates; developability; formulation; linkers; nucleic acids; payloads; site-specific conjugation.
Conflict of interest statement
At the time this manuscript was prepared, all authors were employees of Eli Lilly and Company. All authors are recipients of Eli Lilly and Company stock grants. The authors declare no conflict of interest.
Figures

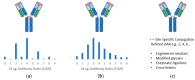

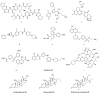
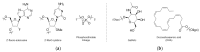
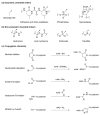

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

