Fabrication and Plasma Modification of Nanofibrous Tissue Engineering Scaffolds
- PMID: 31936372
- PMCID: PMC7023287
- DOI: 10.3390/nano10010119
Fabrication and Plasma Modification of Nanofibrous Tissue Engineering Scaffolds
Abstract
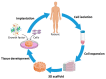
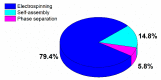
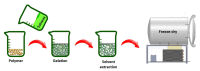
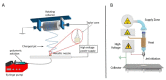
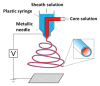
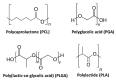
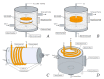
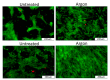
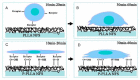
This paper provides a comprehensive overview of nanofibrous structures for tissue engineering purposes and the role of non-thermal plasma technology (NTP) within this field. Special attention is first given to nanofiber fabrication strategies, including thermally-induced phase separation, molecular self-assembly, and electrospinning, highlighting their strengths, weaknesses, and potentials. The review then continues to discuss the biodegradable polyesters typically employed for nanofiber fabrication, while the primary focus lies on their applicability and limitations. From thereon, the reader is introduced to the concept of NTP and its application in plasma-assisted surface modification of nanofibrous scaffolds. The final part of the review discusses the available literature on NTP-modified nanofibers looking at the impact of plasma activation and polymerization treatments on nanofiber wettability, surface chemistry, cell adhesion/proliferation and protein grafting. As such, this review provides a complete introduction into NTP-modified nanofibers, while aiming to address the current unexplored potentials left within the field.
Keywords: electrospun nanofibers; non-thermal plasma treatment; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
-
- O’brien F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today. 2011;14:88–95.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

