Thermo-Mechanical Behaviour of Human Nasal Cartilage
- PMID: 31936593
- PMCID: PMC7023433
- DOI: 10.3390/polym12010177
Thermo-Mechanical Behaviour of Human Nasal Cartilage
Abstract
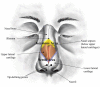
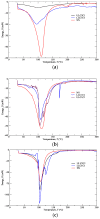
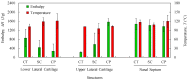
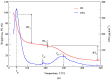
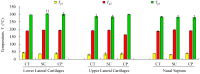
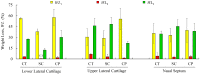
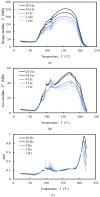
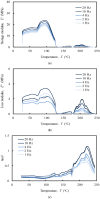
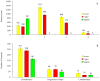
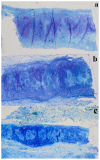
The aim of this study was to undergo a comprehensive analysis of the thermo-mechanical properties of nasal cartilages for the future design of a composite polymeric material to be used in human nose reconstruction surgery. A thermal and dynamic mechanical analysis (DMA) in tension and compression modes within the ranges 1 to 20 Hz and 30 °C to 250 °C was performed on human nasal cartilage. Differential scanning calorimetry (DSC), as well as characterization of the nasal septum (NS), upper lateral cartilages (ULC), and lower lateral cartilages (LLC) reveals the different nature of the binding water inside the studied specimens. Three peaks at 60-80 °C, 100-130 °C, and 200 °C were attributed to melting of the crystalline region of collagen matrix, water evaporation, and the strongly bound non-interstitial water in the cartilage and composite specimens, respectively. Thermogravimetric analysis (TGA) showed that the degradation of cartilage, composite, and subcutaneous tissue of the NS, ULC, and LLC take place in three thermal events (~37 °C, ~189 °C, and ~290 °C) showing that cartilage releases more water and more rapidly than the subcutaneous tissue. The water content of nasal cartilage was estimated to be 42 wt %. The results of the DMA analyses demonstrated that tensile mode is ruled by flow-independent behaviour produced by the time-dependent deformability of the solid cartilage matrix that is strongly frequency-dependent, showing an unstable crystalline region between 80-180 °C, an amorphous region at around 120 °C, and a clear glass transition point at 200 °C (780 kJ/mol). Instead, the unconfined compressive mode is clearly ruled by a flow-dependent process caused by the frictional force of the interstitial fluid that flows within the cartilage matrix resulting in higher stiffness (from 12 MPa at 1 Hz to 16 MPa at 20 Hz in storage modulus). The outcomes of this study will support the development of an artificial material to mimic the thermo-mechanical behaviour of the natural cartilage of the human nose.
Keywords: cartilage; nasal soft tissue; rhinoplasty; thermo-mechanical characterization; viscoelasticity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

