VAPB ER-Aggregates, A Possible New Biomarker in ALS Pathology
- PMID: 31936602
- PMCID: PMC7017080
- DOI: 10.3390/cells9010164
VAPB ER-Aggregates, A Possible New Biomarker in ALS Pathology
Abstract
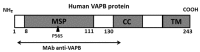
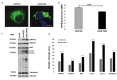
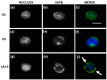
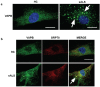
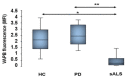
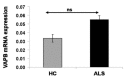
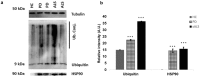
A point mutation (P56S) in the gene-encoding vesicle-associated membrane-protein-associated protein B (VAPB) leads to an autosomal-dominant form of amyotrophic lateral sclerosis (ALS), classified as ALS-8. The mutant VAPB is characterized by ER-associated aggregates that lead to a complete reorganization of ER structures. Growing evidences suggest VAPB involvement in ALS pathomechanisms. In fact, numerous studies demonstrated VAPB alteration also in sporadic ALS (sALS) and showed the presence of its aggregates when others ALS-related gene are mutant. Recently, the identification of new biomarkers in peripheral blood mononuclear cells (PBMCs) has been proposed as a good noninvasive option for studying ALS. Here, we evaluated VAPB as a possible ALS pathologic marker analyzing PBMCs of sALS patients. Immunofluorescence analysis (IFA) showed a peculiar pattern of VAPB aggregates in sALS, not evident in healthy control (HC) subjects and in Parkinson's disease (PD) PBMCs. This specific pattern led us to suppose that VAPB could be misfolded in sALS. The data indirectly confirmed by flow cytometry assay (FCA) showed a reduction of VAPB fluorescent signals in sALS. However, our observations were not associated with the presence of a genetic mutation or altered gene expression of VAPB. Our study brings further evidences of the VAPB role in ALS as a diagnostic biomarker.
Keywords: ALS; PBMC; VAPB ER-aggregates; biomarker; endoplasmic reticulum; flow cytometry; immunofluorescence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Ince P.G., Highley J.R., Kirby J., Wharton S.B., Takahashi H., Strong M.J., Shaw P.J. Molecular pathology and genetic advances in amyotrophic lateral sclerosis: An emerging molecular pathway and the significance of glial pathology. Acta Neuropathol. 2011;122:657–671. doi: 10.1007/s00401-011-0913-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

