Study of Ecophysiological Responses of the Antarctic Fruticose Lichen Cladonia borealis Using the PAM Fluorescence System under Natural and Laboratory Conditions
- PMID: 31936612
- PMCID: PMC7020452
- DOI: 10.3390/plants9010085
Study of Ecophysiological Responses of the Antarctic Fruticose Lichen Cladonia borealis Using the PAM Fluorescence System under Natural and Laboratory Conditions
Abstract
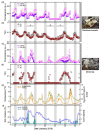
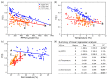
Antarctic lichens have been used as indicators of climate change for decades, but only a few species have been studied. We assessed the photosynthetic performance of the fruticose lichen Cladonia borealis under natural and laboratory conditions using the PAM fluorescence system. Compared to that of sun-adapted Usnea sp., the photosynthetic performance of C. borealis exhibits shade-adapted lichen features, and its chlorophyll fluorescence does not occur during dry days without rain. To understand its desiccation-rehydration responses, we measured changes in the PSII photochemistry in C. borealis under the average light intensity of dawn light and daylight and the desiccating conditions of its natural microclimate. Interestingly, samples under daylight and rapid-desiccation conditions showed a delayed reduction in Fv'/Fm' and rETRmax, and an increase in Y(II) and Y(NPQ) levels. These results suggest that the photoprotective mechanism of C. borealis depends on sunlight and becomes more efficient with improved desiccation tolerance. Amplicon sequencing revealed that the major photobiont of C. borealis was Asterochloris irregularis, which has not been reported in Antarctica before. Collectively, these results from both field and laboratory could provide a better understanding of specific ecophysiological responses of shade-adapted lichens in the Antarctic region.
Keywords: Antarctic; Cladonia borealis; desiccated state; fruticose lichens; non-photochemical quenching; phytochemistry; poikilohydric; shade-adapted lichen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bewley J.D. Physiological Aspects of Desiccation Tolerance. Annu. Rev. Plant Physiol. 1979;30:195–238. doi: 10.1146/annurev.pp.30.060179.001211. - DOI
-
- Rundel P.W. Handbook of Lichenology. Volume 2. CRC Press, Inc.; Boca Raton, FL, USA: 1988. Water relations; pp. 17–36.
-
- Green T.A., Schroeter B., Sancho L.G. Functional Plant Ecology. CRC Press, Inc.; Boca Raton, FL, USA: 2007. Plant life in Antarctica; pp. 408–453.
-
- Rudolph E.D. Biometeorogy, Proceedings of the Third International Biometeorological Congress. Volume 2. Pergamon Press; Oxford, UK: 1966. Lichen ecology and microclimate studies at Cape Hallett, Antarctica; pp. 900–910.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

