HB-EGF-EGFR Signaling in Bone Marrow Endothelial Cells Mediates Angiogenesis Associated with Multiple Myeloma
- PMID: 31936715
- PMCID: PMC7017291
- DOI: 10.3390/cancers12010173
HB-EGF-EGFR Signaling in Bone Marrow Endothelial Cells Mediates Angiogenesis Associated with Multiple Myeloma
Abstract
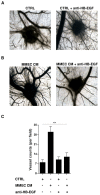
Epidermal growth factor receptor (EGFR) and its ligand heparin-binding EGF-like growth factor (HB-EGF) sustain endothelial cell proliferation and angiogenesis in solid tumors, but little is known about the role of HB-EGF-EGFR signaling in bone marrow angiogenesis and multiple myeloma (MM) progression. We found that bone marrow endothelial cells from patients with MM express high levels of EGFR and HB-EGF, compared with cells from patients with monoclonal gammopathy of undetermined significance, and that overexpressed HB-EGF stimulates EGFR expression in an autocrine loop. We also found that levels of EGFR and HB-EGF parallel MM plasma cell number, and that HB-EGF is a potent inducer of angiogenesis in vitro and in vivo. Moreover, blockade of HB-EGF-EGFR signaling, by an anti-HB-EGF neutralizing antibody or the EGFR inhibitor erlotinib, limited the angiogenic potential of bone marrow endothelial cells and hampered tumor growth in an MM xenograft mouse model. These results identify HB-EGF-EGFR signaling as a potential target of anti-angiogenic therapy, and encourage the clinical investigation of EGFR inhibitors in combination with conventional cytotoxic drugs as a new therapeutic strategy for MM.
Keywords: EGFR; HB-EGF; bone marrow angiogenesis; endothelial cells; multiple myeloma.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures






Similar articles
-
Cypermethrin induces astrocyte apoptosis by the disruption of the autocrine/paracrine mode of epidermal growth factor receptor signaling.Toxicol Sci. 2012 Feb;125(2):473-87. doi: 10.1093/toxsci/kfr303. Epub 2011 Nov 1. Toxicol Sci. 2012. PMID: 22048644
-
Both Autocrine Signaling and Paracrine Signaling of HB-EGF Enhance Ocular Neovascularization.Arterioscler Thromb Vasc Biol. 2018 Jan;38(1):174-185. doi: 10.1161/ATVBAHA.117.310337. Epub 2017 Nov 30. Arterioscler Thromb Vasc Biol. 2018. PMID: 29191924
-
Transactivation of epidermal growth factor receptor after heparin-binding epidermal growth factor-like growth factor shedding in the migration of prostate cancer cells promoted by bombesin.Prostate. 2003 Nov 1;57(3):187-95. doi: 10.1002/pros.10295. Prostate. 2003. PMID: 14518027
-
Heparin-binding epidermal growth factor-like growth factor: p91 activation induction of plasminogen activator/inhibitor, and tubular morphogenesis in human microvascular endothelial cells.Jpn J Cancer Res. 1996 Jan;87(1):68-77. doi: 10.1111/j.1349-7006.1996.tb00202.x. Jpn J Cancer Res. 1996. PMID: 8609052 Free PMC article.
-
Heparin-Binding Epidermal Growth Factor-Like Growth Factor as a Potent Target for Breast Cancer Therapy.Cancer Biother Radiopharm. 2016 Apr;31(3):85-90. doi: 10.1089/cbr.2015.1956. Cancer Biother Radiopharm. 2016. PMID: 27093342 Review.
Cited by
-
High Expression of the Lysosomal Protease Cathepsin D Confers Better Prognosis in Neuroblastoma Patients by Contrasting EGF-Induced Neuroblastoma Cell Growth.Int J Mol Sci. 2022 Apr 26;23(9):4782. doi: 10.3390/ijms23094782. Int J Mol Sci. 2022. PMID: 35563171 Free PMC article.
-
Associations of ANGPT2 expression and its variants (rs1868554 and rs7825407) with multiple myeloma risk and outcome.Front Oncol. 2025 Mar 6;15:1468373. doi: 10.3389/fonc.2025.1468373. eCollection 2025. Front Oncol. 2025. PMID: 40115011 Free PMC article.
-
Direct thrombin inhibitors as alternatives to heparin to preserve lung growth and function in a murine model of compensatory lung growth.Sci Rep. 2022 Dec 7;12(1):21117. doi: 10.1038/s41598-022-25773-3. Sci Rep. 2022. PMID: 36477689 Free PMC article.
-
Decoding the Intricate Landscape of Pancreatic Cancer: Insights into Tumor Biology, Microenvironment, and Therapeutic Interventions.Cancers (Basel). 2024 Jul 2;16(13):2438. doi: 10.3390/cancers16132438. Cancers (Basel). 2024. PMID: 39001498 Free PMC article. Review.
-
AURKA targeting: a NEAT approach to halt myeloma.Haematologica. 2024 Dec 1;109(12):3847-3849. doi: 10.3324/haematol.2024.286085. Haematologica. 2024. PMID: 39219507 Free PMC article. No abstract available.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

