Liquid Chromatography Tandem Mass Spectrometry Quantification of 13C-Labeling in Sugars
- PMID: 31936723
- PMCID: PMC7022953
- DOI: 10.3390/metabo10010030
Liquid Chromatography Tandem Mass Spectrometry Quantification of 13C-Labeling in Sugars
Abstract
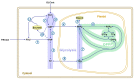
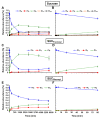
Subcellular compartmentation has been challenging in plant 13C-metabolic flux analysis. Indeed, plant cells are highly compartmented: they contain vacuoles and plastids in addition to the regular organelles found in other eukaryotes. The distinction of reactions between compartments is possible when metabolites are synthesized in a particular compartment or by a unique pathway. Sucrose is an example of such a metabolite: it is specifically produced in the cytosol from glucose 6-phosphate (G6P) and fructose 6-phosphate (F6P). Therefore, determining the 13C-labeling in the fructosyl and glucosyl moieties of sucrose directly informs about the labeling of cytosolic F6P and G6P, respectively. To date, the most commonly used method to monitor sucrose labeling is by nuclear magnetic resonance, which requires substantial amounts of biological sample. This study describes a new methodology that accurately measures the labeling in free sugars using liquid chromatography tandem mass spectrometry (LC-MS/MS). For this purpose, maize embryos were pulsed with [U-13C]-fructose, intracellular sugars were extracted, and their time-course labeling was analyzed by LC-MS/MS. Additionally, extracts were enzymatically treated with hexokinase to remove the soluble hexoses, and then invertase to cleave sucrose into fructose and glucose. Finally, the labeling in the glucosyl and fructosyl moieties of sucrose was determined by LC-MS/MS.
Keywords: 13C-labeling; 13C-metabolic flux analysis; LC-MS/MS; fructose 6-phosphate; glucose 6-phosphate; hexokinase; invertase; subcellular compartmentation; sucrose.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Gas chromatography-mass spectrometry analysis of 13C labeling in sugars for metabolic flux analysis.Anal Biochem. 2012 Jun 15;425(2):183-8. doi: 10.1016/j.ab.2012.03.020. Epub 2012 Apr 1. Anal Biochem. 2012. PMID: 22475504
-
13C labeling analysis of sugars by high resolution-mass spectrometry for metabolic flux analysis.Anal Biochem. 2017 Jun 15;527:45-48. doi: 10.1016/j.ab.2017.02.005. Epub 2017 Feb 14. Anal Biochem. 2017. PMID: 28213171
-
Designer labels for plant metabolism: statistical design of isotope labeling experiments for improved quantification of flux in complex plant metabolic networks.Mol Biosyst. 2013 Jan 27;9(1):99-112. doi: 10.1039/c2mb25253h. Epub 2012 Nov 1. Mol Biosyst. 2013. PMID: 23114423
-
Metabolic flux analysis of secondary metabolism in plants.Metab Eng Commun. 2020 Feb 1;10:e00123. doi: 10.1016/j.mec.2020.e00123. eCollection 2020 Jun. Metab Eng Commun. 2020. PMID: 32099803 Free PMC article. Review.
-
Forced degradation and impurity profiling: recent trends in analytical perspectives.J Pharm Biomed Anal. 2013 Dec;86:11-35. doi: 10.1016/j.jpba.2013.07.013. Epub 2013 Jul 31. J Pharm Biomed Anal. 2013. PMID: 23969330 Review.
Cited by
-
Non-conventional pathways enable pennycress (Thlaspi arvense L.) embryos to achieve high efficiency of oil biosynthesis.J Exp Bot. 2020 May 30;71(10):3037-3051. doi: 10.1093/jxb/eraa060. J Exp Bot. 2020. PMID: 32006014 Free PMC article.
-
Progress in understanding and improving oil content and quality in seeds.Front Plant Sci. 2023 Jan 26;14:1116894. doi: 10.3389/fpls.2023.1116894. eCollection 2023. Front Plant Sci. 2023. PMID: 36778708 Free PMC article. Review.
-
The Glucagon-Like Adipokinetic Hormone in Drosophila melanogaster - Biosynthesis and Secretion.Front Physiol. 2021 Dec 23;12:710652. doi: 10.3389/fphys.2021.710652. eCollection 2021. Front Physiol. 2021. PMID: 35002748 Free PMC article. Review.
-
Multiscale physiological responses to nitrogen supplementation of maize hybrids.Plant Physiol. 2024 Apr 30;195(1):879-899. doi: 10.1093/plphys/kiad583. Plant Physiol. 2024. PMID: 37925649 Free PMC article.
-
Marrubium alysson L. Ameliorated Methotrexate-Induced Testicular Damage in Mice through Regulation of Apoptosis and miRNA-29a Expression: LC-MS/MS Metabolic Profiling.Plants (Basel). 2022 Sep 3;11(17):2309. doi: 10.3390/plants11172309. Plants (Basel). 2022. PMID: 36079691 Free PMC article.
References
-
- Hill S.A., ap Rees T. Fluxes of carbohydrate metabolism in ripening bananas. Planta. 1993;192:52–60. doi: 10.1007/BF00198692. - DOI
-
- Salon C., Raymond P., Pradet A. Quantification of carbon fluxes through the tricarboxylic acid cycle in early germinating lettuce embryos. J. Biol. Chem. 1988;263:12278–12287. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

