Synthetic DNA Vaccines Adjuvanted with pIL-33 Drive Liver-Localized T Cells and Provide Protection from Plasmodium Challenge in a Mouse Model
- PMID: 31936739
- PMCID: PMC7157753
- DOI: 10.3390/vaccines8010021
Synthetic DNA Vaccines Adjuvanted with pIL-33 Drive Liver-Localized T Cells and Provide Protection from Plasmodium Challenge in a Mouse Model
Abstract
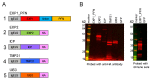
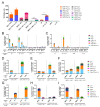
The need for a malaria vaccine is indisputable. A single vaccine for Plasmodium pre-erythrocytic stages targeting the major sporozoite antigen circumsporozoite protein (CSP) has had partial success. Additionally, CD8+ T cells targeting liver-stage (LS) antigens induced by live attenuated sporozoite vaccines were associated with protection in human challenge experiments. To further evaluate protection mediated by LS antigens, we focused on exported pre-erythrocytic proteins (exported protein 1 (EXP1), profilin (PFN), exported protein 2 (EXP2), inhibitor of cysteine proteases (ICP), transmembrane protein 21 (TMP21), and upregulated in infective sporozoites-3 (UIS3)) expressed in all Plasmodium species and designed optimized, synthetic DNA (synDNA) immunogens. SynDNA antigen cocktails were tested with and without the molecular adjuvant plasmid IL-33. Immunized animals developed robust T cell responses including induction of antigen-specific liver-localized CD8+ T cells, which were enhanced by the co-delivery of plasmid IL-33. In total, 100% of mice in adjuvanted groups and 71%-88% in non-adjuvanted groups were protected from blood-stage disease following Plasmodium yoelii sporozoite challenge. This study supports the potential of synDNA LS antigens as vaccine components for malaria parasite infection.
Keywords: DNA vaccines; Plasmodium; exported proteins; liver stage; malaria.
Conflict of interest statement
The authors declare the following conflicts of interest: D.B.W. reports receiving commercial research funding from Inovio, Geneos, and GeneOne; has received speakers bureau honoraria from AstraZeneca, Roche and Merck; has ownership interest in Inovio; and is a consultant/board member for Inovio. L.H. is an employee of Inovio Pharmaceuticals. No potential conflicts of interests were disclosed by the other authors.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

