An Efficient Photodynamic Therapy Treatment for Human Pancreatic Adenocarcinoma
- PMID: 31936786
- PMCID: PMC7019594
- DOI: 10.3390/jcm9010192
An Efficient Photodynamic Therapy Treatment for Human Pancreatic Adenocarcinoma
Abstract
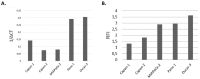
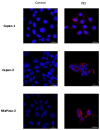
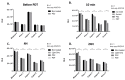
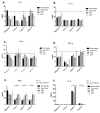
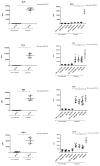
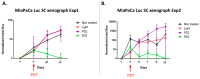
To date, pancreatic adenocarcinoma (ADKP) is a devastating disease for which the incidence rate is close to the mortality rate. The survival rate has evolved only 2-5% in 45 years, highlighting the failure of current therapies. Otherwise, the use of photodynamic therapy (PDT), based on the use of an adapted photosensitizer (PS) has already proved its worth and has prompted a growing interest in the field of oncology. We have developed a new photosensitizer (PS-FOL/PS2), protected by a recently published patent (WO2019 016397-A1, 24 January 2019). This photosensitizer is associated with an addressing molecule (folic acid) targeting the folate receptor 1 (FOLR1) with a high affinity. Folate binds to FOLR1, in a specific way, expressed in 100% of ADKP or over-expressed in 30% of cases. The first objective of this study is to evaluate the effectiveness of this PS2-PDT in four ADKP cell lines: Capan-1, Capan-2, MiapaCa-2, and Panc-1. For this purpose, we first evaluated the gene and protein expression of FOLR1 on four ADKP cell lines. Subsequently, we evaluated PS2's efficacy in our cell lines and we assessed the impact of PDT on the secretome of cancer cells and its impact on the immune system. Finally, we evaluate the PDT efficacy on a humanized SCID mouse model of pancreatic cancer. In a very interesting way, we observed a significant increase in the proliferation of activated-human PBMC when cultured with conditioned media of ADKP cancer cells subjected to PDT. Furthermore, to evaluate in vivo the impact of this new PS, we analyzed the tumor growth in a humanized SCID mice model of pancreatic cancer. Four conditions were tested: Untreated, mice (nontreated), mice with PS (PS2), mice subjected to illumination (Light only), and mice subjected to illumination in the presence of PS (PDT). We noticed that the mice subjected to PDT presented a strong decrease in the growth of the tumor over time after illumination. Our investigations have not only suggested that PS2-PDT is an effective therapy in the treatment of PDAC but also that it activates the immune system and could be considered as a real adjuvant for anti-cancer vaccination. Thus, this new study provides new treatment options for patients in a therapeutic impasse and will provide a new arsenal in the fight against PDAC.
Keywords: folate-coupled photosensitizer; immuno-adjuvant; pancreatic cancer; photodynamic therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ducreux M., Caramella C., Hollebecque A., Burtin P., Goéré D., Seufferlein T., Haustermans K., Van Laethem J.L., Conroy T., Arnold D. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015;26:v56–v68. doi: 10.1093/annonc/mdv295. - DOI - PubMed
-
- Buscail L., Bournet B. Adénocarcinome du pancréas: Quels traitements proposer en 2017 et quelles sont les thérapeutiques d’avenir? La lettre de l’hépato-gastroentérologue. 2017;2:84–88.
LinkOut - more resources
Full Text Sources

