Design of Crosslinked Hydrogels Comprising Poly(Vinylphosphonic Acid) and Bis[2-(Methacryloyloxy)Ethyl] Phosphate as an Efficient Adsorbent for Wastewater Dye Removal
- PMID: 31936837
- PMCID: PMC7023366
- DOI: 10.3390/nano10010131
Design of Crosslinked Hydrogels Comprising Poly(Vinylphosphonic Acid) and Bis[2-(Methacryloyloxy)Ethyl] Phosphate as an Efficient Adsorbent for Wastewater Dye Removal
Abstract
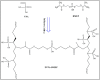
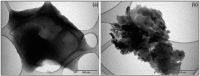
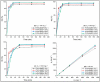
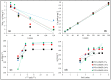
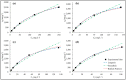
The development of adsorbents with high adsorption capacity and fast separation is of utmost importance for the environmental management of dye-bearing wastewaters. Within this scope, crosslinked hydrogels including poly(vinylphosphonic acid) (PVPA) and bis[2-(methacryloyloxy)ethyl] phosphate (BMEP) were designed with varying mole ratios of BMEP (5-40%). The Fourier transform infrared (FT-IR) spectroscopy, thermogravimetric analysis (TGA), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and Brunauer-Emmett-Teller (BET) results revealed that the fabrication of crosslinked PVPA-BMEP hydrogels enhanced: (i) functionalities of PA groups in the structure of hydrogels, (ii) thermal stabilities up to 250 °C, and (iii) interaction between methylene blue (MB) molecules and hydrogels. The pseudo second-order kinetic model best described the experimental adsorption data. The behaviors of the isotherms were more appropriate for Langmuir than Freundlich isotherm for the experimental data. PVPA-BMEP (40%) hydrogel indicated a fast and an outstanding MB adsorption capacity of 2841 mg g-1, which has not been reported yet for polymer hydrogels, to the best of our knowledge. The thermodynamic studies concluded that MB adsorption process was spontaneous and exothermic in nature. The overall results suggest that the designed and fabricated PVPA-BMEP hydrogels have great potential for the efficient removal of coloring materials from wastewaters.
Keywords: adsorption isotherm; adsorption kinetic; methylene blue adsorption; poly(vinylphosphonic acid); wastewater dye removal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Zargar B., Parham H., Rezazade M. Fast removal and recovery of methylene blue by activated carbon modified with magnetic iron oxide nanoparticles. J. Chin. Chem. Soc. 2011;58:694–699. doi: 10.1002/jccs.201190108. - DOI
-
- Sakin Omer O., Hussein M.A., Hussein B.H.M., Mgaidi A. Adsorption thermodynamics of cationic dyes (methylene blue and crystal violet) to a natural clay mineral from aqueous solution between 293.15 and 323.15 K. Arab. J. Chem. 2018;11:615–623. doi: 10.1016/j.arabjc.2017.10.007. - DOI
-
- Ramezani S., Zahedi P., Bahrami S.-H., Nemati Y. Microfluidic fabrication of nanoparticles based on ethyl acrylate-functionalized chitosan for adsorption of methylene blue from aqueous solutions. J. Polym. Environ. 2019;27:1653–1665. doi: 10.1007/s10924-019-01463-6. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

