Block-And-Lock Strategies to Cure HIV Infection
- PMID: 31936859
- PMCID: PMC7019976
- DOI: 10.3390/v12010084
Block-And-Lock Strategies to Cure HIV Infection
Abstract
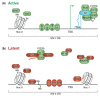
Today HIV infection cannot be cured due to the presence of a reservoir of latently infected cells inducing a viral rebound upon treatment interruption. Hence, the latent reservoir is considered as the major barrier for an HIV cure. So far, efforts to completely eradicate the reservoir via a shock-and-kill approach have proven difficult and unsuccessful. Therefore, more research has been done recently on an alternative block-and-lock functional cure strategy. In contrast to the shock-and-kill strategy that aims to eradicate the entire reservoir, block-and-lock aims to permanently silence all proviruses, even after treatment interruption. HIV silencing can be achieved by targeting different factors of the transcription machinery. In this review, we first describe the underlying mechanisms of HIV transcription and silencing. Next, we give an overview of the different block-and-lock strategies under investigation.
Keywords: HIV; block-and-lock; cure; latency.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Finzi D., Hermankova M., Pierson T., Carruth L.M., Buck C., Chaisson R.E., Quinn T.C., Chadwick K., Margolick J., Brookmeyer R., et al. Identification of a Reservoir for HIV-1 in Patients on Highly Active Antiretroviral Therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. - DOI - PubMed
-
- Finzi D., Blankson J., Siliciano J.D., Margolick J.B., Chadwick K., Pierson T., Smith K., Lisziewica J., Lori F., Flexner C., et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 1999;5:512. doi: 10.1038/8394. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

