Ketone Synthesis from Benzyldiboronates and Esters: Leveraging α-Boryl Carbanions for Carbon-Carbon Bond Formation
- PMID: 31937102
- PMCID: PMC7047910
- DOI: 10.1021/jacs.9b11944
Ketone Synthesis from Benzyldiboronates and Esters: Leveraging α-Boryl Carbanions for Carbon-Carbon Bond Formation
Abstract
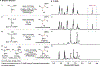
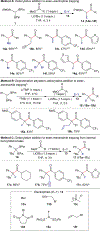
An alkoxide-promoted method for the synthesis of ketones from readily available esters and benzyldiboronates is described. The synthetic method is compatible with a host of sterically differentiated alkyl groups, alkenes, acidic protons α to carbonyl groups, tertiary amides, and aryl rings having common organic functional groups. With esters bearing α-stereocenters, high enantiomeric excess was maintained during ketone formation, establishing minimal competing racemization by deprotonation. Monitoring the reaction between benzyldiboronate and LiOtBu in THF at 23 °C allowed for the identification of products arising from deborylation to form an α-boryl carbanion, deprotonation, and alkoxide addition to form an "-ate" complex. Addition of 4-trifluoromethylbenzoate to this mixture established the α-boryl carbanion as the intermediate responsible for C-C bond formation and ultimately ketone synthesis. Elucidation of the role of this intermediate leveraged additional bond-forming chemistry and enabled the one-pot synthesis of ketones with α-halogen atoms and quaternary centers with four-different carbon substituents.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

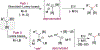


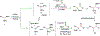
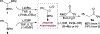
References
-
- Brown HC; Singaram B The Development of a Simple General Procedure for Synthesis of Pure Enantiomers via Chiral Organoboranes. Acc. Chem. Res 1988, 21, 287–293.
- Miyaura N; Suzuki A Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev 1995, 95, 2457–2483.
- Neeve EC; Geier SJ; Mkhalid IAI; Westcott SA; Marder TB Diboron(4) Compounds: From Structural Curiosity to Synthetic Workhorse. Chem. Rev 2016, 116, 9091–9161. - PubMed
- Fyfe JWB; Watson AJB Recent Developments in Organoboron Chemistry: Old Dogs, New Tricks. Chem 2017, 3, 31–55.
- Hartwig JF Borylation and Silylation of C–H Bonds: A Platform for Diverse C–H Bond Functionalizations. Acc. Chem. Res 2012, 45, 864–873. - PubMed
- Hall DG Boronic Acids: Preparation, Applications in Organic Synthesis and Medicine, 2nd ed.; Wiley-VCH: Weinheim, 2011.
-
- Nallagonda R; Padala K; Masarwa A Gem-Diborylalkanes: Recent Advances in Their Preparation, Transformation and Application. Org. Biomol. Chem 2018, 16, 1050–1064. - PubMed
- Abu Ali H; Dembitsky VM; Srebnik M Recent Developments in Bisdiborane Chemistry: B-C-B, B-C-C-B, B-C=C-B, and B-C≡C-B Compounds and Their Biological Applications. Stud. Inorg. Chem 2005, 22, 59–117.
-
- Endo K; Ohkubo T; Hirokami M; Shibata T Chemoselective and Regiospecific Suzuki Coupling on a Multisubstituted sp3-Carbon in 1,1-Diborylalkanes at Room Temperature. J. Am. Chem. Soc 2010, 132, 11033–11035. - PubMed
- Lee JCH; McDonald R; Hall DG Enantioselective Preparation and Chemoselective Cross-Coupling of 1,1-Diboron Compounds. Nat. Chem 2011, 3, 894–899. - PubMed
-
- Joannou MV; Moyer BS; Goldfogel MJ; Meek SJ Silver(I)-Catalyzed Diastereoselective Synthesis of Anti-1,2-Hydroxyboronates. Angew. Chem. Int. Ed 2015, 54, 14141–14145. - PubMed
- Matteson DS; Moody RJ Carbanions from Deprotonation of gem-Diboronic Esters. J. Am. Chem. Soc 1977, 99, 3196–3197.
- Matteson DS; Moody RJ Deprotonation of 1,1-Diboronic Esters and Reactions of the Carbanions with Alkyl Halides and Carbonyl Compounds. Organometallics 1982, 1, 20–28.
-
- Wommack AJ; Kingsbury JS On the Scope of the Pt-Catalyzed Srebnik Diborylation of Diazoalkanes. An Efficient Approach to Chiral Tertiary Boronic Esters and Alcohols via B-Stabilized Carbanions. Tetrahedron Lett. 2014, 55, 3163–3166.
- Hong K; Liu X; Morken JP Simple Access to Elusive α-Boryl Carbanions and Their Alkylation: An Umpolung Construction for Organic Synthesis. J. Am. Chem. Soc 2014, 136, 10581–10584. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

