MRI differences between MOG antibody disease and AQP4 NMOSD
- PMID: 31937191
- PMCID: PMC7363520
- DOI: 10.1177/1352458519893093
MRI differences between MOG antibody disease and AQP4 NMOSD
Abstract
Background: MOG antibody and AQP4 antibody seropositive diseases are immunologically distinct subtypes of neuromyelitis optica spectrum disorders (NMOSD) with similar clinical presentations. MRI findings can be instrumental in distinguishing MOG antibody disease from AQP4 antibody NMOSD.
Objectives: The aim of this study is to characterize the neuroradiological differences between MOG antibody disease and AQP4 antibody NMOSD with the aim to distinguish between the two entities.
Methods: This is a retrospective study of 26 MOG and 25 AQP4 seropositive patients in which MRI features of the brain, spinal cord, and orbit were compared.
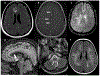
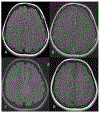
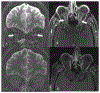
Results: The majority of the abnormal findings in the MOG cohort were located on orbital MRIs, while spinal cord magnetic resonance (MR) abnormalities were more common in the AQP4 cohort. Brain abnormalities showed some overlap, but cortical gray/juxtacortical white matter involvement was distinct to MOG patients, while area postrema involvement was a rare feature.
Conclusion: Cortical gray/juxtacortical white matter lesions on brain MRI might help distinguish MOG antibody disease from AQP4-positive NMOSD. These findings could be of value in distinguishing the two entities as early as the first presentation.
Keywords: AQP4 antibody; MOG antibody; NMOSD; magnetic resonance imaging.
Conflict of interest statement
None of the authors has any conflict of interests to disclose.
Figures





References
-
- Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflammation. 2016;13(1):279. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

