AMG-176, an Mcl-1 Antagonist, Shows Preclinical Efficacy in Chronic Lymphocytic Leukemia
- PMID: 31937611
- PMCID: PMC7358119
- DOI: 10.1158/1078-0432.CCR-19-1397
AMG-176, an Mcl-1 Antagonist, Shows Preclinical Efficacy in Chronic Lymphocytic Leukemia
Abstract
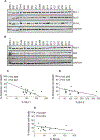
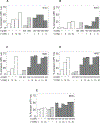
Purpose: Survival of CLL cells due to the presence of Bcl-2 and Mcl-1 has been established. Direct inhibition of Bcl-2 by venetoclax and indirect targeting of Mcl-1 with transcription inhibitors have been successful approaches for CLL. AMG-176 is a selective and direct antagonist of Mcl-1, which has shown efficacy in several hematologic malignancies; however, its effect on CLL is elusive. We evaluated biological and molecular effects of AMG-176 in primary CLL cells.
Experimental design: Using samples from patients (n = 74) with CLL, we tested effects of AMG-176 on CLL and normal hematopoietic cell death and compared importance of CLL prognostic factors on this biological activity. We evaluated CLL cell apoptosis in the presence of stromal cells and identified cell death pathway including stabilization of Mcl-1 protein. Finally, we tested a couplet of AMG-176 and venetoclax in CLL lymphocytes.
Results: AMG-176 incubations resulted in time- and dose-dependent CLL cell death. At 100 and 300 nmol/L, there was 30% and 45% cell death at 24 hours. These concentrations did not result in significant cell death in normal hematopoietic cells. Presence of stroma did not affect AMG-176-induced CLL cell death. IGHV unmutated status, high β2M and Mcl-1 protein levels resulted in slightly lower cell death. Mcl-1, but not Bcl-2 protein levels, in CLL cells increased with AMG-176. Low concentrations of venetoclax (1-30 nmol/L) were additive or synergistic with AMG-176.
Conclusions: AMG-176 is active in inducing CLL cell death while sparing normal blood cells. Combination with low-dose venetoclax was additive or synergistic.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures




Similar articles
-
Splicing modulation sensitizes chronic lymphocytic leukemia cells to venetoclax by remodeling mitochondrial apoptotic dependencies.JCI Insight. 2018 Oct 4;3(19):e121438. doi: 10.1172/jci.insight.121438. JCI Insight. 2018. PMID: 30282833 Free PMC article.
-
Therapeutic targeting of apoptosis in chronic lymphocytic leukemia.Semin Hematol. 2024 Apr;61(2):109-118. doi: 10.1053/j.seminhematol.2024.01.015. Epub 2024 Feb 7. Semin Hematol. 2024. PMID: 38538512 Review.
-
Pathways and mechanisms of venetoclax resistance.Leuk Lymphoma. 2017 Sep;58(9):1-17. doi: 10.1080/10428194.2017.1283032. Epub 2017 Jan 31. Leuk Lymphoma. 2017. PMID: 28140720 Free PMC article. Review.
-
Venetoclax: Bcl-2 inhibition for the treatment of chronic lymphocytic leukemia.Drugs Today (Barc). 2016 Apr;52(4):249-60. doi: 10.1358/dot.2016.52.4.2470954. Drugs Today (Barc). 2016. PMID: 27252989 Review.
-
Pairing MCL-1 inhibition with venetoclax improves therapeutic efficiency of BH3-mimetics in AML.Eur J Haematol. 2020 Nov;105(5):588-596. doi: 10.1111/ejh.13492. Epub 2020 Aug 4. Eur J Haematol. 2020. PMID: 32659848
Cited by
-
KS18, a Mcl-1 inhibitor, improves the effectiveness of bortezomib and overcomes resistance in refractory multiple myeloma by triggering intrinsic apoptosis.Front Pharmacol. 2024 Oct 1;15:1436786. doi: 10.3389/fphar.2024.1436786. eCollection 2024. Front Pharmacol. 2024. PMID: 39411073 Free PMC article.
-
Mcl-1 Inhibition: Managing Malignancy in Multiple Myeloma.Front Pharmacol. 2021 Jul 19;12:699629. doi: 10.3389/fphar.2021.699629. eCollection 2021. Front Pharmacol. 2021. PMID: 34349655 Free PMC article. Review.
-
Indoleamine 2, 3-Dioxygenase 1 Mediates Survival Signals in Chronic Lymphocytic Leukemia via Kynurenine/Aryl Hydrocarbon Receptor-Mediated MCL1 Modulation.Front Immunol. 2022 Mar 18;13:832263. doi: 10.3389/fimmu.2022.832263. eCollection 2022. Front Immunol. 2022. PMID: 35371054 Free PMC article.
-
AMG176, an MCL-1 inhibitor, is active in pre-clinical models of aggressive B-cell lymphomas.Leuk Lymphoma. 2023 Jun;64(6):1175-1185. doi: 10.1080/10428194.2023.2200876. Epub 2023 Apr 19. Leuk Lymphoma. 2023. PMID: 37074033 Free PMC article.
-
Cyclin-dependent kinase inhibitor fadraciclib (CYC065) depletes anti-apoptotic protein and synergizes with venetoclax in primary chronic lymphocytic leukemia cells.Leukemia. 2022 Jun;36(6):1596-1608. doi: 10.1038/s41375-022-01553-w. Epub 2022 Apr 5. Leukemia. 2022. PMID: 35383271 Free PMC article.
References
-
- Birkinshaw RW, Czabotar PE. The BCL-2 family of proteins and mitochondrial outer membrane permeabilisation. 2017. Elsevier; p 152–62. - PubMed
-
- Lessene G, Czabotar PE, Colman PM. BCL-2 family antagonists for cancer therapy. Nature reviews Drug discovery 2008;7(12):989. - PubMed
-
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005;435(7042):677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

