A Quantitative Centrosomal Amplification Score Predicts Local Recurrence of Ductal Carcinoma In Situ
- PMID: 31937618
- PMCID: PMC7299818
- DOI: 10.1158/1078-0432.CCR-19-1272
A Quantitative Centrosomal Amplification Score Predicts Local Recurrence of Ductal Carcinoma In Situ
Abstract
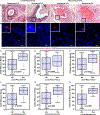
Purpose: The purpose of this study is to predict risk of local recurrence (LR) in ductal carcinoma in situ (DCIS) with a new visualization and quantification approach using centrosome amplification (CA), a cancer cell-specific trait widely associated with aggressiveness.
Experimental design: This first-of-its-kind methodology evaluates the severity and frequency of numerical and structural CA present within DCIS and assigns a quantitative centrosomal amplification score (CAS) to each sample. Analyses were performed in a discovery cohort (DC, n = 133) and a validation cohort (VC, n = 119).
Results: DCIS cases with LR exhibited significantly higher CAS than recurrence-free cases. Higher CAS was associated with a greater risk of developing LR (HR, 6.3 and 4.8 for DC and VC, respectively; P < 0.001). CAS remained an independent predictor of relapse-free survival (HR, 7.4 and 4.5 for DC and VC, respectively; P < 0.001) even after accounting for potentially confounding factors [grade, age, comedo necrosis, and radiotherapy (RT)]. Patient stratification using CAS (P < 0.0001) was superior to that by Van Nuys Prognostic Index (VNPI; HR for CAS = 6.2 vs. HR for VNPI = 1.1). Among patients treated with breast-conserving surgery alone, CAS identified patients likely to benefit from adjuvant RT.
Conclusions: CAS predicted 10-year LR risk for patients who underwent surgical management alone and identified patients who may be at low risk of recurrence, and for whom adjuvant RT may not be required. CAS demonstrated the highest concordance among the known prognostic models such as VNPI and clinicopathologic variables such as grade, age, and comedo necrosis.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures


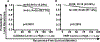
Similar articles
-
Prognostic Significance of Clinicopathologic Features in Patients With Breast Ductal Carcinoma-in-Situ Who Received Breast-Conserving Surgery.Clin Breast Cancer. 2018 Dec;18(6):441-450.e2. doi: 10.1016/j.clbc.2018.04.002. Epub 2018 Apr 10. Clin Breast Cancer. 2018. PMID: 29709458
-
259 Patients with DCIS of the breast applying USC/Van Nuys prognostic index: a retrospective review with long term follow up.Breast Cancer Res Treat. 2008 Jun;109(3):405-16. doi: 10.1007/s10549-007-9668-7. Epub 2007 Aug 9. Breast Cancer Res Treat. 2008. PMID: 17687650 Review.
-
Therapeutic strategy for ductal carcinoma in situ patients according to Van Nuys Prognostic Index.Ann Ital Chir. 2014 May-Jun;85(3):254-9. Ann Ital Chir. 2014. PMID: 25074533
-
Multidisciplinary Shared Decision Making in the Management of Ductal Carcinoma In Situ of the Breast.Ann Surg Oncol. 2015 Dec;22 Suppl 3(0 3):S516-21. doi: 10.1245/s10434-015-4607-z. Epub 2015 May 19. Ann Surg Oncol. 2015. PMID: 25986869 Free PMC article.
-
[Evaluation of predictive factors, particularly the Van Nuys index, of local recurrence in ductal carcinoma in situ of the breast: study of 166 cases with conservative treatment and review of the literature].Bull Cancer. 2001 Apr;88(4):419-25. Bull Cancer. 2001. PMID: 11371378 Review. French.
Cited by
-
Centrosome amplification: a quantifiable cancer cell trait with prognostic value in solid malignancies.Cancer Metastasis Rev. 2021 Mar;40(1):319-339. doi: 10.1007/s10555-020-09937-z. Epub 2020 Oct 26. Cancer Metastasis Rev. 2021. PMID: 33106971 Free PMC article. Review.
-
Prognostic model based on centrosome-related genes constructed in head and neck squamous cell carcinoma.J Cancer. 2024 Oct 21;15(20):6531-6544. doi: 10.7150/jca.102057. eCollection 2024. J Cancer. 2024. PMID: 39668833 Free PMC article.
-
2-methoxyestradiol sensitizes breast cancer cells to taxanes by targeting centrosomes.Oncotarget. 2020 Dec 1;11(48):4479-4489. doi: 10.18632/oncotarget.27810. eCollection 2020 Dec 1. Oncotarget. 2020. PMID: 33400733 Free PMC article.
References
-
- Page DL, Dupont WD, Rogers LW, Landenberger M. Intraductal carcinoma of the breast: follow-up after biopsy only. Cancer 1982;49(4):751–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

