A Bispecific Antibody That Simultaneously Recognizes the V2- and V3-Glycan Epitopes of the HIV-1 Envelope Glycoprotein Is Broader and More Potent than Its Parental Antibodies
- PMID: 31937648
- PMCID: PMC6960291
- DOI: 10.1128/mBio.03080-19
A Bispecific Antibody That Simultaneously Recognizes the V2- and V3-Glycan Epitopes of the HIV-1 Envelope Glycoprotein Is Broader and More Potent than Its Parental Antibodies
Abstract
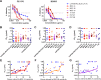
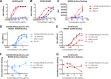
Broadly neutralizing antibodies (bNAbs) can prevent and control an HIV-1 infection, but their breadth is invariably too limited for use as monotherapy. To address this problem, bi- and trispecific antibody-like constructs have been developed. These engineered antibodies typically have greater breadth than the native bNAbs from which they were derived, but they are not more potent because they do not, in most cases, simultaneously engage more than a single epitope of the HIV-1 envelope glycoprotein (Env). Here, we describe a new class of bispecific antibodies targeting the V2-glycan (apex) and V3-glycan regions of the HIV-1 envelope glycoprotein (Env). Specifically, bispecific antibodies with a single-chain (scFv) form of the CAP256.VRC26.25 V2-glycan (apex) antibody on one antibody arm and a full V3-glycan Fab on the other arm neutralizes more HIV-1 isolates than the bNAbs from which they were derived. Moreover, these bispecific antibodies are markedly more potent than their parental bNAbs, likely because they simultaneously engage both the apex and V3-glycan epitopes of Env. Our data show that simultaneous engagement of two critical epitopes of a single Env trimer can markedly increase the potency of a bispecific antibody.IMPORTANCE Broadly neutralizing antibodies (bNAbs) can prevent a new HIV-1 infection and can at least temporarily suppress an established infection. However, antibody-resistant viruses rapidly emerge in infected persons treated with any single bNAb. Several bispecific antibodies have been developed to increase the breadth of these antibodies, but typically only one arm of these bispecific constructs binds the HIV-1 envelope glycoprotein trimer (Env). Here, we develop and characterize bispecific constructs based on well-characterized V2-glycan and V3-glycan bNAbs and show that at least one member of this class is more potent than its parental antibodies, indicating that they can simultaneously bind both of these epitopes of a single Env trimer. These data show that bispecific antibody-like proteins can achieve greater neutralization potency than the bNAbs from which they were derived.
Keywords: antibody neutralization; bispecific antibodies; broadly neutralizing antibodies; human immunodeficiency virus; human immunodeficiency virus 1.
Copyright © 2020 Davis-Gardner et al.
Figures




Similar articles
-
Targeting the HIV-1 Spike and Coreceptor with Bi- and Trispecific Antibodies for Single-Component Broad Inhibition of Entry.J Virol. 2018 Aug 29;92(18):e00384-18. doi: 10.1128/JVI.00384-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29976677 Free PMC article.
-
A Rare Mutation in an Infant-Derived HIV-1 Envelope Glycoprotein Alters Interprotomer Stability and Susceptibility to Broadly Neutralizing Antibodies Targeting the Trimer Apex.J Virol. 2020 Sep 15;94(19):e00814-20. doi: 10.1128/JVI.00814-20. Print 2020 Sep 15. J Virol. 2020. PMID: 32669335 Free PMC article.
-
Functional Stability of HIV-1 Envelope Trimer Affects Accessibility to Broadly Neutralizing Antibodies at Its Apex.J Virol. 2017 Nov 30;91(24):e01216-17. doi: 10.1128/JVI.01216-17. Print 2017 Dec 15. J Virol. 2017. PMID: 28978711 Free PMC article.
-
HIV-1 immunogens and strategies to drive antibody responses towards neutralization breadth.Retrovirology. 2018 Nov 26;15(1):74. doi: 10.1186/s12977-018-0457-7. Retrovirology. 2018. PMID: 30477581 Free PMC article. Review.
-
Stabilizing HIV-1 envelope glycoprotein trimers to induce neutralizing antibodies.Retrovirology. 2018 Sep 12;15(1):63. doi: 10.1186/s12977-018-0445-y. Retrovirology. 2018. PMID: 30208933 Free PMC article. Review.
Cited by
-
Bispecific antibody CAP256.J3LS targets V2-apex and CD4-binding sites with high breadth and potency.MAbs. 2023 Jan-Dec;15(1):2165390. doi: 10.1080/19420862.2023.2165390. MAbs. 2023. PMID: 36729903 Free PMC article.
-
Bispecific antibodies targeting two glycoproteins on SFTSV exhibit synergistic neutralization and protection in a mouse model.Proc Natl Acad Sci U S A. 2024 Jun 11;121(24):e2400163121. doi: 10.1073/pnas.2400163121. Epub 2024 Jun 3. Proc Natl Acad Sci U S A. 2024. PMID: 38830098 Free PMC article.
-
Long-acting HIV pre-exposure prophylaxis (PrEP) approaches: recent advances, emerging technologies, and development challenges.Expert Opin Drug Deliv. 2022 Oct;19(10):1365-1380. doi: 10.1080/17425247.2022.2135699. Epub 2022 Oct 25. Expert Opin Drug Deliv. 2022. PMID: 36252277 Free PMC article. Review.
-
Engineering SARS-CoV-2 cocktail antibodies into a bispecific format improves neutralizing potency and breadth.bioRxiv [Preprint]. 2022 Feb 1:2022.02.01.478504. doi: 10.1101/2022.02.01.478504. bioRxiv. 2022. Update in: Nat Commun. 2022 Sep 22;13(1):5552. doi: 10.1038/s41467-022-33284-y. PMID: 35132410 Free PMC article. Updated. Preprint.
-
Engineering antibody-based molecules for HIV treatment and cure.Curr Opin HIV AIDS. 2020 Sep;15(5):290-299. doi: 10.1097/COH.0000000000000640. Curr Opin HIV AIDS. 2020. PMID: 32732551 Free PMC article. Review.
References
-
- Burton DR, Barbas CF III, Persson MA, Koenig S, Chanock RM, Lerner RA. 1991. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci U S A 88:10134–10137. doi:10.1073/pnas.88.22.10134. - DOI - PMC - PubMed
-
- Buchacher A, Predl R, Strutzenberger K, Steinfellner W, Trkola A, Purtscher M, Gruber G, Tauer C, Steindl F, Jungbauer A. 1994. Generation of human monoclonal antibodies against HIV-1 proteins: electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res Hum Retroviruses 10:359–369. doi:10.1089/aid.1994.10.359. - DOI - PubMed
-
- Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412. doi:10.1038/nature11544. - DOI - PMC - PubMed
-
- Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. 2011. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333:1633–1637. doi:10.1126/science.1207227. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

