Treatment of human skeletal muscle cells with inhibitors of diacylglycerol acyltransferases 1 and 2 to explore isozyme-specific roles on lipid metabolism
- PMID: 31937853
- PMCID: PMC6959318
- DOI: 10.1038/s41598-019-57157-5
Treatment of human skeletal muscle cells with inhibitors of diacylglycerol acyltransferases 1 and 2 to explore isozyme-specific roles on lipid metabolism
Abstract
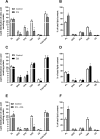
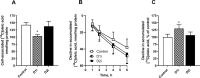
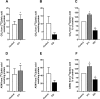
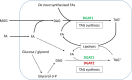
Diacylglycerol acyltransferases (DGAT) 1 and 2 catalyse the final step in triacylglycerol (TAG) synthesis, the esterification of fatty acyl-CoA to diacylglycerol. Despite catalysing the same reaction and being present in the same cell types, they exhibit different functions on lipid metabolism in various tissues. Yet, their roles in skeletal muscle remain poorly defined. In this study, we investigated how selective inhibitors of DGAT1 and DGAT2 affected lipid metabolism in human primary skeletal muscle cells. The results showed that DGAT1 was dominant in human skeletal muscle cells utilizing fatty acids (FAs) derived from various sources, both exogenously supplied FA, de novo synthesised FA, or FA derived from lipolysis, to generate TAG, as well as being involved in de novo synthesis of TAG. On the other hand, DGAT2 seemed to be specialised for de novo synthesis of TAG from glycerol-3-posphate only. Interestingly, DGAT activities were also important for regulating FA oxidation, indicating a key role in balancing FAs between storage in TAG and efficient utilization through oxidation. Finally, we observed that inhibition of DGAT enzymes could potentially alter glucose-FA interactions in skeletal muscle. In summary, treatment with DGAT1 or DGAT2 specific inhibitors resulted in different responses on lipid metabolism in human myotubes, indicating that the two enzymes play distinct roles in TAG metabolism in skeletal muscle.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

