Glycofullerenes as non-receptor tyrosine kinase inhibitors- towards better nanotherapeutics for pancreatic cancer treatment
- PMID: 31937861
- PMCID: PMC6959220
- DOI: 10.1038/s41598-019-57155-7
Glycofullerenes as non-receptor tyrosine kinase inhibitors- towards better nanotherapeutics for pancreatic cancer treatment
Abstract
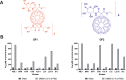
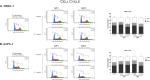
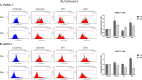
The water-soluble glycofullerenes GF1 and GF2 were synthesized using two-step modified Bingel-Hirsch methodology. Interestingly, we identified buckyballs as a novel class of non-receptor Src kinases inhibitors. The evaluated compounds were found to inhibit Fyn A and BTK proteins with IC50 values in the low micromolar range, with the most active compound at 39 µM. Moreover, we have demonstrated that formation of protein corona on the surface of [60]fullerene derivatives is changing the landscape of their activity, tuning the selectivity of obtained carbon nanomaterials towards Fyn A and BTK kinases. The performed molecular biology studies revealed no cytotoxicity and no influence of engineered carbon nanomaterials on the cell cycle of PANC-1 and AsPC-1 cancer cell lines. Incubation with the tested compounds resulted in the cellular redox imbalance triggering the repair systems and influenced the changing of protein levels.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Kang S.-g., Zhou G., Yang P., Liu Y., Sun B., Huynh T., Meng H., Zhao L., Xing G., Chen C., Zhao Y., Zhou R. Molecular mechanism of pancreatic tumor metastasis inhibition by Gd@C82(OH)22 and its implication for de novo design of nanomedicine. Proceedings of the National Academy of Sciences. 2012;109(38):15431–15436. doi: 10.1073/pnas.1204600109. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

