Single-cell transcriptional profiles in human skeletal muscle
- PMID: 31937892
- PMCID: PMC6959232
- DOI: 10.1038/s41598-019-57110-6
Single-cell transcriptional profiles in human skeletal muscle
Abstract
Skeletal muscle is a heterogeneous tissue comprised of muscle fiber and mononuclear cell types that, in addition to movement, influences immunity, metabolism and cognition. We investigated the gene expression patterns of skeletal muscle cells using RNA-seq of subtype-pooled single human muscle fibers and single cell RNA-seq of mononuclear cells from human vastus lateralis, mouse quadriceps, and mouse diaphragm. We identified 11 human skeletal muscle mononuclear cell types, including two fibro-adipogenic progenitor (FAP) cell subtypes. The human FBN1+ FAP cell subtype is novel and a corresponding FBN1+ FAP cell type was also found in single cell RNA-seq analysis in mouse. Transcriptome exercise studies using bulk tissue analysis do not resolve changes in individual cell-type proportion or gene expression. The cell-type gene signatures provide the means to use computational methods to identify cell-type level changes in bulk studies. As an example, we analyzed public transcriptome data from an exercise training study and revealed significant changes in specific mononuclear cell-type proportions related to age, sex, acute exercise and training. Our single-cell expression map of skeletal muscle cell types will further the understanding of the diverse effects of exercise and the pathophysiology of muscle disease.
Conflict of interest statement
The authors declare no competing interests.
Figures



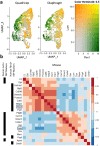
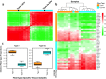
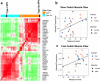

References
-
- Saltin, B. & Gollnick, P. D. in Handbook of Physiology: Skeletal Muscle (eds. Peachy, L. D., Adnan, R. & Geiger, S. R.) 555–631 (1983).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

