Antimalarial N 1, N 3-Dialkyldioxonaphthoimidazoliums: Synthesis, Biological Activity, and Structure-activity Relationships
- PMID: 31938463
- PMCID: PMC6956359
- DOI: 10.1021/acsmedchemlett.9b00457
Antimalarial N 1, N 3-Dialkyldioxonaphthoimidazoliums: Synthesis, Biological Activity, and Structure-activity Relationships
Abstract
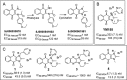
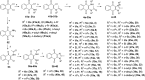
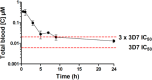
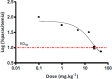
Here we report the nanomolar potencies of N 1,N 3-dialkyldioxonaphthoimidazoliums against asexual forms of sensitive and resistant Plasmodium falciparum. Activity was dependent on the presence of the fused quinone-imidazolium entity and lipophilicity imparted by the N1/N3 alkyl residues on the scaffold. Gametocytocidal activity was also detected, with most members active at IC50 < 1 μM. A representative analog with good solubility, limited PAMPA permeability, and microsomal stability demonstrated oral efficacy on a humanized mouse model of P. falciparum.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- World Malaria Report 2018; World Health Organization: Geneva, 2018.
-
- Hamilton W. L.; Amato R.; van der Pluijm R. W.; Jacob C. G.; Quang H. H.; Thuy-Nhien N. T.; Hien T. T.; Hongvanthong B.; Chindavongsa K.; Mayxay M.; Huy R.; Leang R.; Huch C.; Dysoley L.; Amaratunga C.; Suon S.; Fairhurst R. M.; Tripura R.; Peto T. J.; Sovann Y.; Jittamala P.; Hanboonkunupakarn B.; Pukrittayakamee S.; Chau N. J.; Imwong M.; Dhorda M.; Vongpromek R.; Chan X. H. S.; Maude R. J.; Pearson R. D.; Nguyen T.; Rockett K.; Drury E.; Goncalves S.; White N. J.; Day N. P.; Kwiatkowski D. P.; Dondorp A. M.; Miotto O. Evolution and expansion of multi-drug resistant malaria in Southeast Asia: a genomic epidemiology study. Lancet Infect. Dis. 2019, 19, 943–951. 10.1016/S1473-3099(19)30392-5. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

