Long-term Efficacy, Safety, and Immunogenicity of the Infliximab (IFX) Biosimilar, PF-06438179/GP1111, in Patients with Rheumatoid Arthritis After Switching from Reference IFX or Continuing Biosimilar Therapy: Week 54-78 Data From a Randomized, Double-Blind, Phase III Trial
- PMID: 31939063
- PMCID: PMC7113200
- DOI: 10.1007/s40259-019-00403-z
Long-term Efficacy, Safety, and Immunogenicity of the Infliximab (IFX) Biosimilar, PF-06438179/GP1111, in Patients with Rheumatoid Arthritis After Switching from Reference IFX or Continuing Biosimilar Therapy: Week 54-78 Data From a Randomized, Double-Blind, Phase III Trial
Abstract
Objective: Our objective was to evaluate the long-term efficacy, safety, and immunogenicity of the infliximab biosimilar, PF-06438179/GP1111 (PF-SZ-IFX), in patients with rheumatoid arthritis (RA) who continued biosimilar treatment throughout 78 weeks or who switched from reference infliximab (Remicade®) sourced from the EU (IFX-EU) at week 30 or week 54 in the REFLECTIONS B537-02 study.
Methods: In this phase III, double-blind, active-controlled study, patients with moderate-to-severe active RA were initially randomized to PF-SZ-IFX or IFX-EU, each with methotrexate (treatment period [TP] 1; N = 650). At week 30, patients receiving PF-SZ-IFX continued PF-SZ-IFX; patients receiving IFX-EU were re-randomized to continue IFX-EU or switch to PF-SZ-IFX (TP2; n = 566). From weeks 54 to 78, all patients received open-label treatment with PF-SZ-IFX (TP3; n = 505). Efficacy, safety, and immunogenicity data were analyzed during TP3.
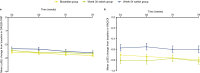
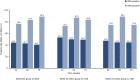
Results: Efficacy was sustained and comparable across groups at week 78, with American College of Rheumatology criteria for ≥ 20% clinical improvement response rates of 75.9% (biosimilar group), 77.8% (week 30 switch group), and 68.3% (week 54 switch group). The incidence of treatment-emergent adverse events was 28.9%, 29.4%, and 30.2%, respectively. The proportion of patients who were antidrug antibody (ADA) positive and neutralizing antibody positive (as a percentage of ADA-positive patients) was stable and comparable between groups.
Conclusions: Results to week 78 continue to support the efficacy, safety, and immunogenicity of PF-SZ-IFX in patients with moderate-to-severe active RA. There were no clinically meaningful differences between groups, independent of a single treatment transition from IFX-EU to PF-SZ-IFX at week 30 or week 54.
Trial registration number: NCT02222493.
Conflict of interest statement
SBC has received research grants and consulting fees or honorarium from AbbVie, Amgen, Pfizer, and Sandoz. RA has no conflicts of interest that are directly relevant to the content of this article. HK has received consulting fees, speaking fees, and/or honoraria from AbbVie GK, Asahi Kasei Pharma, Bristol-Myers Squibb, Chugai Pharmaceutical, Eli Lilly Japan KK, Janssen Pharmaceutical KK, Mitsubishi Tanabe Pharma, Novartis, and Pfizer Japan and has received research grants from AbbVie GK, Asahi Kasei Pharma, Astellas Pharma, Chugai Pharmaceutical, Eisai, Mitsubishi Tanabe Pharma, and Novartis. AJK has received consulting fees and/or speaking fees and/or fees for participation on advisory committees or review panels from Sanofi, Regeneron, SUN Pharma Advanced Research, AbbVie, Janssen, Pfizer, UCB, Genzyme, Boehringer-Ingelheim, Celgene, Horizon, Merck, Novartis, and Flexion and has stock holdings for Pfizer, Sanofi, Amgen, and Gilead. MT has received research grants, speaking fees, and honoraria from Pfizer Philippines. SCR has received research grants from Pfizer. OvR is an employee of Hexal AG (a Sandoz company). CC, SH, MIR, and MZ are employees of, and hold stock or stock options from, Pfizer.
Figures


Similar articles
-
Randomised, double-blind, phase III study comparing the infliximab biosimilar, PF-06438179/GP1111, with reference infliximab: efficacy, safety and immunogenicity from week 30 to week 54.RMD Open. 2019 Mar 28;5(1):e000876. doi: 10.1136/rmdopen-2018-000876. eCollection 2019. RMD Open. 2019. PMID: 30997153 Free PMC article. Clinical Trial.
-
A randomized controlled trial comparing PF-06438179/GP1111 (an infliximab biosimilar) and infliximab reference product for treatment of moderate to severe active rheumatoid arthritis despite methotrexate therapy.Arthritis Res Ther. 2018 Jul 27;20(1):155. doi: 10.1186/s13075-018-1646-4. Arthritis Res Ther. 2018. PMID: 30053896 Free PMC article. Clinical Trial.
-
A comparative study of PF-06438179/GP1111 (an infliximab biosimilar) and reference infliximab in patients with moderate to severe active rheumatoid arthritis: A subgroup analysis.Int J Rheum Dis. 2020 Jul;23(7):876-881. doi: 10.1111/1756-185X.13846. Epub 2020 May 31. Int J Rheum Dis. 2020. PMID: 32476277 Free PMC article. Clinical Trial.
-
Infliximab biosimilar GP1111: a review of 5 years' post-approval experience.Expert Opin Biol Ther. 2024 Jul;24(7):615-625. doi: 10.1080/14712598.2024.2377298. Epub 2024 Jul 27. Expert Opin Biol Ther. 2024. PMID: 38976286 Review.
-
PF-06438179/GP1111: An Infliximab Biosimilar.BioDrugs. 2018 Dec;32(6):639-642. doi: 10.1007/s40259-018-0310-5. BioDrugs. 2018. PMID: 30284704 Free PMC article. Review.
Cited by
-
Use of multibiomarker disease activity scores in biosimilarity studies for the treatment of patients with rheumatoid arthritis.RMD Open. 2022 Sep;8(2):e002423. doi: 10.1136/rmdopen-2022-002423. RMD Open. 2022. PMID: 36180101 Free PMC article.
-
Regulatory Evaluation of Biosimilars: Refinement of Principles Based on the Scientific Evidence and Clinical Experience.BioDrugs. 2022 May;36(3):359-371. doi: 10.1007/s40259-022-00533-x. Epub 2022 May 21. BioDrugs. 2022. PMID: 35596890 Free PMC article. Review.
-
Efficacy and Safety of CMAB008 Compared with Innovator Infliximab in Patients with Moderate-to-Severe Rheumatoid Arthritis Receiving Concomitant Methotrexate: A Randomized, Double-blind, Multi-center, Phase III Non-inferiority Study.Rheumatol Ther. 2023 Jun;10(3):757-773. doi: 10.1007/s40744-023-00544-2. Epub 2023 Mar 25. Rheumatol Ther. 2023. PMID: 36964872 Free PMC article.
-
Safety outcomes when switching between biosimilars and reference biologics: A systematic review and meta-analysis.PLoS One. 2023 Oct 3;18(10):e0292231. doi: 10.1371/journal.pone.0292231. eCollection 2023. PLoS One. 2023. PMID: 37788264 Free PMC article.
-
Assessing MAPPs assay as a tool to predict the immunogenicity potential of protein therapeutics.Life Sci Alliance. 2023 Oct 13;7(1):e202302095. doi: 10.26508/lsa.202302095. Print 2024 Jan. Life Sci Alliance. 2023. PMID: 37833075 Free PMC article.
References
-
- Janssen Biotech Inc. Remicade® (infliximab) US prescribing information. 2013. http://www.remicade.com/shared/product/remicade/prescribing-information.pdf. Accessed 31 Oct 2018.
-
- European Medicines Agency. Remicade (infliximab). Summary of Product Characteristics. 2009. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Info.... Accessed 20 Aug 2018.
-
- Janssen Biotech Inc. Official website for Remicade® (infliximab). 2017. https://www.remicade.com/. Accessed 20 Aug 2018.
-
- Nam JL, Winthrop KL, van Vollenhoven RF, Pavelka K, Valesini G, Hensor EM, et al. Current evidence for the management of rheumatoid arthritis with biological disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of RA. Ann Rheum Dis. 2010;69(6):976–986. doi: 10.1136/ard.2009.126573. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

