Catechol-functionalized hydrogels: biomimetic design, adhesion mechanism, and biomedical applications
- PMID: 31939475
- PMCID: PMC7208057
- DOI: 10.1039/c9cs00285e
Catechol-functionalized hydrogels: biomimetic design, adhesion mechanism, and biomedical applications
Abstract
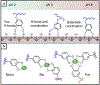
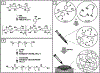
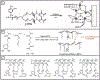
Hydrogels are a unique class of polymeric materials that possess an interconnected porous network across various length scales from nano- to macroscopic dimensions and exhibit remarkable structure-derived properties, including high surface area, an accommodating matrix, inherent flexibility, controllable mechanical strength, and excellent biocompatibility. Strong and robust adhesion between hydrogels and substrates is highly desirable for their integration into and subsequent performance in biomedical devices and systems. However, the adhesive behavior of hydrogels is severely weakened by the large amount of water that interacts with the adhesive groups reducing the interfacial interactions. The challenges of developing tough hydrogel-solid interfaces and robust bonding in wet conditions are analogous to the adhesion problems solved by marine organisms. Inspired by mussel adhesion, a variety of catechol-functionalized adhesive hydrogels have been developed, opening a door for the design of multi-functional platforms. This review is structured to give a comprehensive overview of adhesive hydrogels starting with the fundamental challenges of underwater adhesion, followed by synthetic approaches and fabrication techniques, as well as characterization methods, and finally their practical applications in tissue repair and regeneration, antifouling and antimicrobial applications, drug delivery, and cell encapsulation and delivery. Insights on these topics will provide rational guidelines for using nature's blueprints to develop hydrogel materials with advanced functionalities and uncompromised adhesive properties.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures



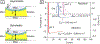

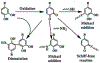







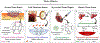


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

