Association of Baseline Prostate-Specific Antigen Level With Long-term Diagnosis of Clinically Significant Prostate Cancer Among Patients Aged 55 to 60 Years: A Secondary Analysis of a Cohort in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial
- PMID: 31940039
- PMCID: PMC6991265
- DOI: 10.1001/jamanetworkopen.2019.19284
Association of Baseline Prostate-Specific Antigen Level With Long-term Diagnosis of Clinically Significant Prostate Cancer Among Patients Aged 55 to 60 Years: A Secondary Analysis of a Cohort in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial
Abstract
Importance: The use of prostate-specific antigen (PSA) screening for prostate cancer is controversial because of the risk of overdiagnosis and overtreatment of indolent cancers. Optimal screening strategies are highly sought.
Objective: To estimate the long-term risk of any prostate cancer and clinically significant prostate cancer based on baseline PSA levels among men aged 55 to 60 years.
Design, setting, and participants: This secondary analysis of a cohort in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial uses actuarial analysis to analyze the association of baseline PSA levels with long-term risk of any prostate cancer and of clinically significant prostate cancer among men aged 55 to 60 years enrolled in the screening group of the trial between 1993 and 2001.
Exposure: Single PSA measurement at study entry.
Main outcomes and measures: Long-term risk of any prostate cancer and clinically significant prostate cancer diagnoses.
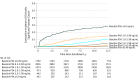
Results: There were 10 968 men aged 55 to 60 years (median [interquartile range] age, 57 [55-58] years) at study enrollment in the screening group of the PLCO Cancer Screening Trial who had long-term follow-up. Actuarial 13-year incidences of clinically significant prostate cancer diagnosis among participants with a baseline PSA of 0.49 ng/mL or less was 0.4% (95% CI, 0%-0.8%); 0.50-0.99 ng/mL, 1.5% (95% CI, 1.1%-1.9%); 1.00-1.99 ng/mL, 5.4% (95% CI, 4.4%-6.4%); 2.00-2.99 ng/mL, 10.6% (95% CI, 8.3%-12.9%); 3.00-3.99 ng/mL, 15.3% (95% CI, 11.4%-19.2%); and 4.00 ng/mL and greater, 29.5% (95% CI, 24.2%-34.8%) (all pairwise log-rank P ≤ .004). Only 15 prostate cancer-specific deaths occurred during 13 years of follow-up, and 9 (60.0%) were among men with a baseline PSA level of 2.00 ng/mL or higher.
Conclusions and relevance: In this secondary analysis of a cohort from the PLCO Cancer Screening Trial, baseline PSA levels among men aged 55 to 60 years were associated with long-term risk of clinically significant prostate cancer. These findings suggest that repeated screening can be less frequent among men aged 55 to 60 years with a low baseline PSA level (ie, <2.00 ng/mL) and possibly discontinued among those with baseline PSA levels of less than 1.00 ng/mL.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

