Ampakine pretreatment enables a single brief hypoxic episode to evoke phrenic motor facilitation
- PMID: 31940229
- PMCID: PMC7099472
- DOI: 10.1152/jn.00708.2019
Ampakine pretreatment enables a single brief hypoxic episode to evoke phrenic motor facilitation
Abstract
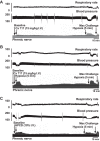
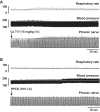
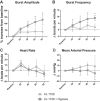
Phrenic long-term facilitation (LTF) is a sustained increase in phrenic motor output occurring after exposure to multiple (but not single) hypoxic episodes. Ampakines are a class of drugs that enhance AMPA receptor function. Ampakines can enhance expression of neuroplasticity, and the phrenic motor system is fundamentally dependent on excitatory glutamatergic currents. Accordingly, we tested the hypothesis that combining ampakine pretreatment with a single brief hypoxic exposure would result in phrenic motor facilitation lasting well beyond the period of hypoxia. Phrenic nerve output was recorded in urethane-anesthetized, ventilated, and vagotomized adult Sprague-Dawley rats. Ampakine CX717 (15 mg/kg iv; n = 8) produced a small increase in phrenic inspiratory burst amplitude and frequency, but values quickly returned to predrug baseline. When CX717 was followed 2 min later by a 5-min exposure to hypoxia (n = 8; ~45 mmHg), a persistent increase in phrenic inspiratory burst amplitude (i.e., phrenic motor facilitation) was observed up to 60 min posthypoxia (103 ± 53% increase from baseline). In contrast, when hypoxia was preceded by vehicle injection (10% 2-hydroxypropyl-β-cyclodextrin; n = 8), inspiratory phrenic bursting was similar to baseline values at 60 min. Additional experiments with another ampakine (CX1739, 15 mg/kg) produced comparable results. We conclude that pairing low-dose ampakine treatment with a single brief hypoxic exposure can evoke sustained phrenic motor facilitation. This targeted approach for enhancing respiratory neuroplasticity may have value in the context of hypoxia-based neurorehabilitation strategies.NEW & NOTEWORTHY A single brief episode of hypoxia (e.g., 3-5 min) does not evoke long-lasting increases in respiratory motor output after the hypoxia is concluded. Ampakines are a class of drugs that enhance AMPA receptor function. We show that pairing low-dose ampakine treatment with a single brief hypoxic exposure can evoke sustained phrenic motor facilitation after the acute hypoxic episode.
Keywords: ampakine; neuroplasticity; respiratory.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




Similar articles
-
Pattern sensitivity of ampakine-hypoxia interactions for evoking phrenic motor facilitation in anesthetized rat.J Neurophysiol. 2024 Feb 1;131(2):216-224. doi: 10.1152/jn.00315.2023. Epub 2023 Dec 20. J Neurophysiol. 2024. PMID: 38116608 Free PMC article.
-
Pharmacological modulation of respiratory control: Ampakines as a therapeutic strategy.Pharmacol Ther. 2025 Jan;265:108744. doi: 10.1016/j.pharmthera.2024.108744. Epub 2024 Nov 8. Pharmacol Ther. 2025. PMID: 39521442 Review.
-
Ampakine pretreatment enables a single hypoxic episode to produce phrenic motor facilitation with no added benefit of additional episodes.J Neurophysiol. 2021 Oct 1;126(4):1420-1429. doi: 10.1152/jn.00307.2021. Epub 2021 Sep 8. J Neurophysiol. 2021. PMID: 34495779 Free PMC article.
-
Ampakines stimulate phrenic motor output after cervical spinal cord injury.Exp Neurol. 2020 Dec;334:113465. doi: 10.1016/j.expneurol.2020.113465. Epub 2020 Sep 17. Exp Neurol. 2020. PMID: 32949571 Free PMC article.
-
The impact of inflammation on respiratory plasticity.Exp Neurol. 2017 Jan;287(Pt 2):243-253. doi: 10.1016/j.expneurol.2016.07.022. Epub 2016 Jul 27. Exp Neurol. 2017. PMID: 27476100 Free PMC article. Review.
Cited by
-
Targeting drug or gene delivery to the phrenic motoneuron pool.J Neurophysiol. 2023 Jan 1;129(1):144-158. doi: 10.1152/jn.00432.2022. Epub 2022 Nov 23. J Neurophysiol. 2023. PMID: 36416447 Free PMC article. Review.
-
Spinally delivered ampakine CX717 increases phrenic motor output in adult rats.Respir Physiol Neurobiol. 2022 Feb;296:103814. doi: 10.1016/j.resp.2021.103814. Epub 2021 Nov 11. Respir Physiol Neurobiol. 2022. PMID: 34775071 Free PMC article.
-
Pattern sensitivity of ampakine-hypoxia interactions for evoking phrenic motor facilitation in anesthetized rat.J Neurophysiol. 2024 Feb 1;131(2):216-224. doi: 10.1152/jn.00315.2023. Epub 2023 Dec 20. J Neurophysiol. 2024. PMID: 38116608 Free PMC article.
-
Pharmacological modulation of respiratory control: Ampakines as a therapeutic strategy.Pharmacol Ther. 2025 Jan;265:108744. doi: 10.1016/j.pharmthera.2024.108744. Epub 2024 Nov 8. Pharmacol Ther. 2025. PMID: 39521442 Review.
-
Ampakines increase diaphragm activation following mid-cervical contusion injury in rats.Exp Neurol. 2024 Jun;376:114769. doi: 10.1016/j.expneurol.2024.114769. Epub 2024 Apr 4. Exp Neurol. 2024. PMID: 38582278 Free PMC article.
References
-
- Arai A, Kessler M, Rogers G, Lynch G. Effects of a memory-enhancing drug on dl-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor currents and synaptic transmission in hippocampus. J Pharmacol Exp Ther 278: 627–638, 1996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

