In vivo expression of peptidylarginine deiminase in Drosophila melanogaster
- PMID: 31940417
- PMCID: PMC6961906
- DOI: 10.1371/journal.pone.0227822
In vivo expression of peptidylarginine deiminase in Drosophila melanogaster
Abstract
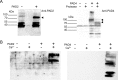
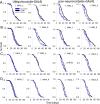
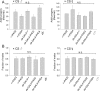
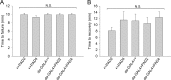
Peptidylarginine deiminase (PAD) modifies peptidylarginine and converts it to peptidylcitrulline in the presence of elevated calcium. Protein modification can lead to severe changes in protein structure and function, and aberrant PAD activity is linked to human pathologies. While PAD homologs have been discovered in vertebrates-as well as in protozoa, fungi, and bacteria-none have been identified in Drosophila melanogaster, a simple and widely used animal model for human diseases. Here, we describe the development of a human PAD overexpression model in Drosophila. We established fly lines harboring human PAD2 or PAD4 transgenes for ectopic expression under control of the GAL4/UAS system. We show that ubiquitous or nervous system expression of PAD2 or PAD4 have minimal impact on fly lifespan, fecundity, and the response to acute heat stress. Although we did not detect citrullinated proteins in fly homogenates, fly-expressed PAD4-but not PAD2-was active in vitro upon Ca2+ supplementation. The transgenic fly lines may be valuable in future efforts to develop animal models of PAD-related disorders and for investigating the biochemistry and regulation of PAD function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Peptidylarginine Deiminase Inhibitor Application, Using Cl-Amidine, PAD2, PAD3 and PAD4 Isozyme-Specific Inhibitors in Pancreatic Cancer Cells, Reveals Roles for PAD2 and PAD3 in Cancer Invasion and Modulation of Extracellular Vesicle Signatures.Int J Mol Sci. 2021 Jan 30;22(3):1396. doi: 10.3390/ijms22031396. Int J Mol Sci. 2021. PMID: 33573274 Free PMC article.
-
Peptidylarginine deiminase expression and activity in PAD2 knock-out and PAD4-low mice.Biochimie. 2013 Feb;95(2):299-308. doi: 10.1016/j.biochi.2012.09.029. Epub 2012 Sep 28. Biochimie. 2013. PMID: 23026755
-
PAD enzymes in rheumatoid arthritis: pathogenic effectors and autoimmune targets.Nat Rev Rheumatol. 2020 Jun;16(6):301-315. doi: 10.1038/s41584-020-0409-1. Epub 2020 Apr 27. Nat Rev Rheumatol. 2020. PMID: 32341463 Review.
-
Citrulline Not a Major Determinant in the Recognition of Peptidylarginine Deiminase 2 and 4 by Autoantibodies in Rheumatoid Arthritis.Arthritis Rheumatol. 2020 Sep;72(9):1476-1482. doi: 10.1002/art.41276. Epub 2020 Jul 14. Arthritis Rheumatol. 2020. PMID: 32255561 Free PMC article.
-
The roles of PAD2- and PAD4-mediated protein citrullination catalysis in cancers.Int J Cancer. 2021 Jan 15;148(2):267-276. doi: 10.1002/ijc.33205. Epub 2020 Aug 8. Int J Cancer. 2021. PMID: 33459350 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

