Neuroinflammation-Associated Aspecific Manipulation of Mouse Predator Fear by Toxoplasma gondii
- PMID: 31940479
- PMCID: PMC6963786
- DOI: 10.1016/j.celrep.2019.12.019
Neuroinflammation-Associated Aspecific Manipulation of Mouse Predator Fear by Toxoplasma gondii
Abstract
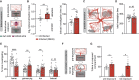
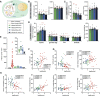
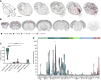
In rodents, the decrease of felid aversion induced by Toxoplasma gondii, a phenomenon termed fatal attraction, is interpreted as an adaptive manipulation by the neurotropic protozoan parasite. With the aim of understanding how the parasite induces such specific behavioral modifications, we performed a multiparametric analysis of T. gondii-induced changes on host behavior, physiology, and brain transcriptome as well as parasite cyst load and distribution. Using a set of complementary behavioral tests, we provide strong evidence that T. gondii lowers general anxiety in infected mice, increases explorative behaviors, and surprisingly alters predator aversion without selectivity toward felids. Furthermore, we show a positive correlation between the severity of the behavioral alterations and the cyst load, which indirectly reflects the level of inflammation during brain colonization. Taken together, these findings refute the myth of a selective loss of cat fear in T. gondii-infected mice and point toward widespread immune-related alterations of behaviors.
Keywords: Apicomplexa; Toxoplasma gondii; cat; chronic infection; effector molecules; host-pathogen interaction; innate behavior; light-sheet microscopy; parasites; predator avoidance.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Arranz-Solís D., Aguado-Martínez A., Müller J., Regidor-Cerrillo J., Ortega-Mora L.M., Hemphill A. Dose-dependent effects of experimental infection with the virulent Neospora caninum Nc-Spain7 isolate in a pregnant mouse model. Vet. Parasitol. 2015;211:133–140. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

