Probiotic Bacillus subtilis Protects against α-Synuclein Aggregation in C. elegans
- PMID: 31940482
- PMCID: PMC6963774
- DOI: 10.1016/j.celrep.2019.12.078
Probiotic Bacillus subtilis Protects against α-Synuclein Aggregation in C. elegans
Abstract
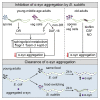
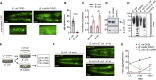
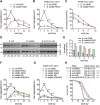
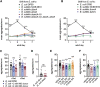
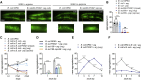
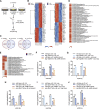
Recent discoveries have implicated the gut microbiome in the progression and severity of Parkinson's disease; however, how gut bacteria affect such neurodegenerative disorders remains unclear. Here, we report that the Bacillus subtilis probiotic strain PXN21 inhibits α-synuclein aggregation and clears preformed aggregates in an established Caenorhabditis elegans model of synucleinopathy. This protection is seen in young and aging animals and is partly mediated by DAF-16. Multiple B. subtilis strains trigger the protective effect via both spores and vegetative cells, partly due to a biofilm formation in the gut of the worms and the release of bacterial metabolites. We identify several host metabolic pathways differentially regulated in response to probiotic exposure, including sphingolipid metabolism. We further demonstrate functional roles of the sphingolipid metabolism genes lagr-1, asm-3, and sptl-3 in the anti-aggregation effect. Our findings provide a basis for exploring the disease-modifying potential of B. subtilis as a dietary supplement.
Keywords: B. subtilis; C. elegans; DAF-16/FOXO; Parkinson’s disease; biofilm; dietary restriction; microbiota; probiotics; sphingolipid metabolism; α-synuclein.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Abbott S.K., Li H., Muñoz S.S., Knoch B., Batterham M., Murphy K.E., Halliday G.M., Garner B. Altered ceramide acyl chain length and ceramide synthase gene expression in Parkinson’s disease. Mov. Disord. 2014;29:518–526. - PubMed
-
- Alafuzoff I., Hartikainen P. Alpha-synucleinopathies. Handb. Clin. Neurol. 2017;145:339–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

