Isolated Anti-HBc: Significance and Management
- PMID: 31940817
- PMCID: PMC7019847
- DOI: 10.3390/jcm9010202
Isolated Anti-HBc: Significance and Management
Abstract
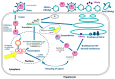
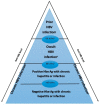
Hepatitis B virus (HBV) infection is prevalent worldwide and is associated with dramatic levels of morbidity and mortality. Isolated anti-HBc (IAHBc) is a particular serological pattern that is commonly found in immunocompromised patients. There is ongoing debate regarding the management of patients with IAHBc. Herein, we summarize the current guidelines and the newest evidence. The frequency of IAHBc is variable, with a higher prevalence in some populations, such as persons living with HIV and others immunocompromised patients. The risk of HBV reactivation depends on host factors (including immunosuppression) and viral factors. It is now well established that immunocompromised patients can be classified into three groups for risk according to the type of immunosuppression and/or treatment. In patients at high risk, HBV therapy has to be considered systematically. In patients at moderate risk, the decision is based on the level of HBV DNA (preemptive treatment or monitoring and vaccination). In patients with low risk, HBV vaccination is another possible approach, although further studies are needed to assess the type of preemptive strategy.
Keywords: HBV reactivation; hepatitis B infection; immunosuppression; isolated anti-HBc antibodies; management; viral hepatitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health Organization . Global Hepatitis Report, 2017. World Health Organization; Genève, Switzerland: 2017. Global Hepatitis Programme.
-
- WHO|Prevention and Control of Viral Hepatitis Infection: Framework for Global Action. [(accessed on 11 August 2019)]; Available online: https://www.who.int/hepatitis/publications/Framework/en/
Publication types
LinkOut - more resources
Full Text Sources

