Cytokine Directed Chondroblast Trans-Differentiation: JAK Inhibition Facilitates Direct Reprogramming of Fibroblasts to Chondroblasts
- PMID: 31940860
- PMCID: PMC7017373
- DOI: 10.3390/cells9010191
Cytokine Directed Chondroblast Trans-Differentiation: JAK Inhibition Facilitates Direct Reprogramming of Fibroblasts to Chondroblasts
Abstract
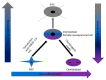
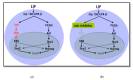
Osteoarthritis (OA) is a degenerative disease of the hyaline articular cartilage. This disease is progressive and may lead to disability. Researchers proposed many regenerative approaches to treat osteoarthritis, including stem cells. Trans-differentiation of a fully differentiated cell state directly into another different differentiated cell state avoids the disadvantages of fully reprogramming cells to induced pluripotent stem cells (iPSCs) in terms of faster reprogramming of the needed cells. Trans-differentiation also reduces the risk of tumor formation by avoiding the iPSC state. OSKM factors (Oct4, Sox2, Klf4, and cMyc) accompanied by the JAK-STAT pathway inhibition, followed by the introduction of specific differentiation factors, directly reprogrammed mouse embryonic fibroblasts to chondroblasts. Our results showed the absence of intermediate induced pluripotent stem cell formation. The resulting aggregates showed clear hyaline and hypertrophic cartilage. Tumor formation was absent in sub-cutaneous capsules transplanted in SCID mice.
Keywords: cartilage regeneration; cellular reprogramming; osteoarthritis; trans-differentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
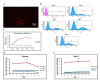
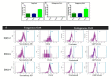

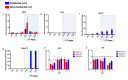

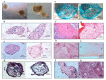
References
-
- Deiana M., Malerba G., Dalle Carbonare L.D., Cheri S., Patuzzo C., Tsenov G., Moron Dalla Tor L.M.D., Mori A., Saviola G., Zipeto D., et al. Physical Activity Prevents Cartilage Degradation: A Metabolomics Study Pinpoints the Involvement of Vitamin B6. Cells. 2019;8:1374. doi: 10.3390/cells8111374. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

