Chemistry and Some Biological Potential of Bismuth and Antimony Dithiocarbamate Complexes
- PMID: 31940910
- PMCID: PMC7024263
- DOI: 10.3390/molecules25020305
Chemistry and Some Biological Potential of Bismuth and Antimony Dithiocarbamate Complexes
Abstract
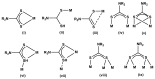
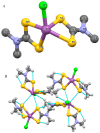
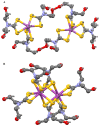
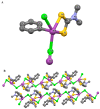
Interest in the synthesis of Bi(III) and Sb(III) dithiocarbamate complexes is on the rise, and this has been attributed to their wide structural diversity and their interesting application as biological agents and in solid state/materials chemistry. The readily available binding sites of the two sulphur atoms within the dithiocarbamate moiety in the complexes confers a wide variety of geometry and interactions that often leads to supramolecular assemblies. Although none of the bismuth or antimony metals are known to play any natural biological function, their dithiocarbamate complexes, however, have proven very useful as antibacterial, antileishmanial, anticancer, and antifungal agents. The dithiocarbamate ligands modulate the associated toxicity of the metals, especially antimony, since bismuth is known to be benign, allowing the metal ion to get to the targeted sites; hence, making it less available for side and other damaging reactions. This review presents a concise chemistry and some known biological potentials of their trivalent dithiocarbamate complexes.
Keywords: antimony; biological activities; bismuth; dithiocarbamate; structural properties.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Garje S., Jain V.K. Chemistry of arsenic, antimony and bismuth compounds derived from xanthate, dithiocarbamate and phosphorus based ligands. Coord. Chem. Rev. 2003;236:35–56. doi: 10.1016/S0010-8545(02)00159-5. - DOI
-
- Tamilvanan S., Gurumoorthy G., Thirumaran S., Ciattini S. Bismuth(III) furfuryl based dithiocarbamates: Synthesis, structures, biological activities and their utility for the preparation of Bi2S3 and Bi2O3 nanoparticles. Polyhedron. 2017;123:111–121. doi: 10.1016/j.poly.2016.10.026. - DOI
-
- Abdullah N.H., Zainal Z., Silong S., Tahir M.I.M., Tan K.-B., Chang S.-K. Thermal decomposition synthesis of nanorods bismuth sulphide from bismuth N-ethyl cyclohexyl dithiocarbamate complex. Thermochim. Acta. 2016;632:37–45. doi: 10.1016/j.tca.2016.03.001. - DOI
-
- Joshi S., Chauhan H.P.S., Carpenter N. Preparation, spectroscopic characterization and antimicrobial activities of mixed metal (Sb and Bi) bridged derivatives with mixed sulfur donor ligands. J. Mol. Struct. 2017;1128:221–229. doi: 10.1016/j.molstruc.2016.08.063. - DOI
-
- Gowda V., Sarma B., Laitinen R.S., Larsson A.-C., Ivanov A.V., Iuga D., Lantto P., Antzutkin O.N. Structural insights into the polymorphism of bismuth(III) di-n-butyldithiocarbamate by X-ray diffraction, solid-state (13C/15N) CP-MAS NMR and DFT calculations. Polyhedron. 2017;129:123–132. doi: 10.1016/j.poly.2017.03.018. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

