Idebenone Protects against Acute Murine Colitis via Antioxidant and Anti-Inflammatory Mechanisms
- PMID: 31940911
- PMCID: PMC7013829
- DOI: 10.3390/ijms21020484
Idebenone Protects against Acute Murine Colitis via Antioxidant and Anti-Inflammatory Mechanisms
Abstract
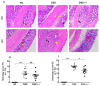
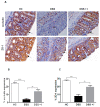
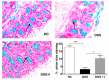
Oxidative stress is a key player of the inflammatory cascade responsible for the initiation of ulcerative colitis (UC). Although the short chain quinone idebenone is considered a potent antioxidant and a mitochondrial electron donor, emerging evidence suggests that idebenone also displays anti-inflammatory activity. This study evaluated the impact of idebenone in the widely used dextran sodium sulphate (DSS)-induced mouse model of acute colitis. Acute colitis was induced in C57BL/6J mice via continuous exposure to 2.5% DSS over 7 days. Idebenone was co-administered orally at a dose of 200 mg/kg body weight. Idebenone significantly prevented body weight loss and improved the disease activity index (DAI), colon length, and histopathological score. Consistent with its reported antioxidant function, idebenone significantly reduced the colonic levels of malondialdehyde (MDA) and nitric oxide (NO), and increased the expression of the redox factor NAD(P)H (nicotinamide adenine dinucleotide phosphate) dehydrogenase quinone-1 (NQO-1) in DSS-exposed mice. Immunohistochemistry revealed a significantly increased expression of tight junction proteins, which protect and maintain paracellular intestinal permeability. In support of an anti-inflammatory activity, idebenone significantly attenuated the elevated levels of pro-inflammatory cytokines in colon tissue. These results suggest that idebenone could represent a promising therapeutic strategy to interfere with disease pathology in UC by simultaneously inducing antioxidative and anti-inflammatory pathways.
Keywords: cytokines; idebenone; inflammatory bowel disease; lipid peroxidation; superoxide dismutase; tight junction proteins and ulcerative colitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Luteolin ameliorates dextran sulfate sodium-induced colitis in mice possibly through activation of the Nrf2 signaling pathway.Int Immunopharmacol. 2016 Nov;40:24-31. doi: 10.1016/j.intimp.2016.08.020. Epub 2016 Aug 28. Int Immunopharmacol. 2016. PMID: 27569028
-
Gallic acid attenuates dextran sulfate sodium-induced experimental colitis in BALB/c mice.Drug Des Devel Ther. 2015 Jul 30;9:3923-34. doi: 10.2147/DDDT.S86345. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26251571 Free PMC article.
-
Pharmacological activity of 6-gingerol in dextran sulphate sodium-induced ulcerative colitis in BALB/c mice.Phytother Res. 2015 Apr;29(4):566-72. doi: 10.1002/ptr.5286. Epub 2015 Jan 29. Phytother Res. 2015. PMID: 25631463
-
Idebenone and neuroprotection: antioxidant, pro-oxidant, or electron carrier?J Bioenerg Biomembr. 2015 Apr;47(1-2):111-8. doi: 10.1007/s10863-014-9571-y. Epub 2014 Sep 28. J Bioenerg Biomembr. 2015. PMID: 25262284 Free PMC article. Review.
-
Berberine Administration in Treatment of Colitis: A Review.Curr Drug Targets. 2020;21(13):1385-1393. doi: 10.2174/1389450121666200621193758. Curr Drug Targets. 2020. PMID: 32564751 Review.
Cited by
-
Anti-Inflammatory Effects of Idebenone Attenuate LPS-Induced Systemic Inflammatory Diseases by Suppressing NF-κB Activation.Antioxidants (Basel). 2024 Jan 25;13(2):151. doi: 10.3390/antiox13020151. Antioxidants (Basel). 2024. PMID: 38397749 Free PMC article.
-
Novel Models for Chronic Intestinal Inflammation in Chickens: Intestinal Inflammation Pattern and Biomarkers.Front Immunol. 2021 May 12;12:676628. doi: 10.3389/fimmu.2021.676628. eCollection 2021. Front Immunol. 2021. PMID: 34054868 Free PMC article.
-
Protection of dystrophic muscle cells using Idebenone correlates with the interplay between calcium, oxidative stress and inflammation.Int J Exp Pathol. 2023 Feb;104(1):4-12. doi: 10.1111/iep.12463. Epub 2022 Dec 24. Int J Exp Pathol. 2023. PMID: 36565155 Free PMC article.
-
Short-Chain Naphthoquinone Protects Against Both Acute and Spontaneous Chronic Murine Colitis by Alleviating Inflammatory Responses.Front Pharmacol. 2021 Aug 23;12:709973. doi: 10.3389/fphar.2021.709973. eCollection 2021. Front Pharmacol. 2021. PMID: 34497514 Free PMC article.
-
Oral Macrocystis pyrifera Fucoidan Administration Exhibits Anti-Inflammatory and Antioxidant Properties and Improves DSS-Induced Colitis in C57BL/6J Mice.Pharmaceutics. 2022 Nov 4;14(11):2383. doi: 10.3390/pharmaceutics14112383. Pharmaceutics. 2022. PMID: 36365201 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

