Proteomic Analysis of Irradiation with Millimeter Waves on Soybean Growth under Flooding Conditions
- PMID: 31940953
- PMCID: PMC7013696
- DOI: 10.3390/ijms21020486
Proteomic Analysis of Irradiation with Millimeter Waves on Soybean Growth under Flooding Conditions
Abstract
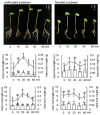
Improving soybean growth and tolerance under environmental stress is crucial for sustainable development. Millimeter waves are a radio-frequency band with a wavelength range of 1-10 mm that has dynamic effects on organisms. To investigate the potential effects of millimeter-waves irradiation on soybean seedlings, morphological and proteomic analyses were performed. Millimeter-waves irradiation improved the growth of roots/hypocotyl and the tolerance of soybean to flooding stress. Proteomic analysis indicated that the irradiated soybean seedlings recovered under oxidative stress during growth, whereas proteins related to glycolysis and ascorbate/glutathione metabolism were not affected. Immunoblot analysis confirmed the promotive effect of millimeter waves to glycolysis- and redox-related pathways under flooding conditions. Sugar metabolism was suppressed under flooding in unirradiated soybean seedlings, whereas it was activated in the irradiated ones, especially trehalose synthesis. These results suggest that millimeter-waves irradiation on soybean seeds promotes the recovery of soybean seedlings under oxidative stress, which positively regulates soybean growth through the regulation of glycolysis and redox related pathways.
Keywords: crop productivity; early-stage soybean; glycolysis; redox; seed irradiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Schutyser M.A.I., Pelgrom P.J.M., Van der Goot A.J., Boom R.M. Dry fractionation for sustainable production of functional legume protein concentrates. Trends Food Sci. Technol. 2015;45:327–335. doi: 10.1016/j.tifs.2015.04.013. - DOI
-
- Schutyser M.A.I., Van der Goot A.J. The potential of dry fractionation processes for sustainable plant protein production. Trends Food Sci. Technol. 2011;22:154–164. doi: 10.1016/j.tifs.2010.11.006. - DOI
-
- Hartman G.L., West E.D., Herman T.K. Crops that feed the world 2. Soybean-worldwide production, use, and constraints caused by pathogens and pests. Food Secur. 2011;3:5–17. doi: 10.1007/s12571-010-0108-x. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

